In 1975, Kohler and Milstein developed the hybridoma technology which fuses antibody-producing B cells with immortal cancer cell lines, which in turn creates an immortal hybridoma cell line that can indefinitely produce antibodies. Hybridoma technology is one of the most widely used techniques in modern research.
Diverse immunization strategies are applied to obtain B cells for different research objectives. The animal will be sacrificed when its serum produces sufficient antibodies.
The spleen of the sacrificed animal is removed, and activated B cells are isolated under aseptic conditions. The antibodies produced by the activated B cells in the serum are identified by assays such as ELISA. The activated B lymphocytes are then fused with myeloma cells.
Metastatic tumor cells are cultured in 8-azacytidine a few weeks prior to cell fusion to obtain the non-functional hypoxanthine-guanine phosphoribosyltransferase (HGPRT) gene in myeloma cells (the preferred method for hybridoma technology).
Cell fusion is the process of fusing activated B lymphocytes with HAT-sensitive myeloma cells. In this step, freshly obtained activated B cells are centrifuged with HAT-sensitive myeloma cells in a fusion-promoting medium. Electrofusion is another method of fusion in which cells fuse in response to an electric field.
Even with the most efficient fusion techniques, only 1–2% of fused hybridoma cells can be produced. Therefore, hybridoma selection is crucial, that is currently performed primarily by incubating cell mixtures for 10–14 days in HAT medium, where the presence of aminopterin blocks the ability of cells to synthesize nucleotides via the de novo synthesis pathway. Hypoxanthine and deoxythymidine enable cells with functional hypoxanthine-guanine phosphoribosyltransferase (HGPRT) genes to survive through the salvage pathway. Therefore, hybrid cells with a functional HGPRT gene from B lymphocytes survive, whereas B cells and malignant neoplastic cells that have not fused are eliminated.
Hybridomas are screened to select ones that produced antibodies against the specific epitope of the antigen.
Cloning and proliferation of hybridoma cells in large culture vessels or flasks.
Monoclonal antibodies can currently be produced both in vitro and in vivo. In vivo, mouse ascites are used to produce monoclonal antibodies, while in vitro, hybridoma cells are cultured and monoclonal antibodies are isolated using culture media.
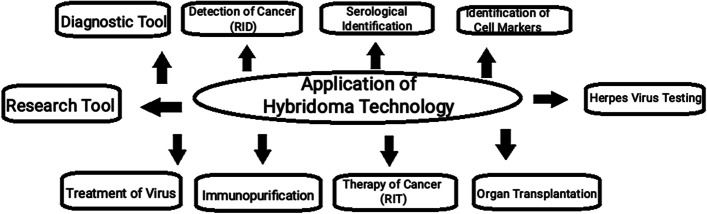 Fig 1. Application of hybridoma technology. (Mitra, S., et al., 2021)
Fig 1. Application of hybridoma technology. (Mitra, S., et al., 2021)
Hybridoma Antigen Preparation Overview
Hybridoma Immunization Strategies Overview
Hybridoma Animal Selection Overview
Hybridoma Cell Screening Overview
Hybridoma Rescue Strategies Overview
Creative Biolabs is pleased to share our expertise and experience in hybridoma to facilitate our clients' research.
Reference
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.