At present, it is important to assess the potential immunogenicity of drugs during drug development to better guide clinical drug delivery while managing the clinical consequences of immunogenicity. Scientists tend to focus on developing the latest diagnostic and chemotherapeutic drugs, but these foreign substances can easily cause immunogenic mediated adverse effects in the subject. Therefore, the evaluation method of immunogenicity came into being. In this field, Creative Biolabs can provide a series of diagnostic services on immunogenicity, extensive information research, and quality technical support to ensure the smooth progress in your testing process.
The strength of immunogenicity is one of the determinants of biotechnology drug development, and immunogenicity testing is critical for evaluating drug safety. ELISA, western blot analysis and surface plasmon resonance SPR technology are all common methods for biopharmaceutical immunogenicity detection. Surface plasmon resonance (SPR) as one of the real-time kinetic binding assays, is to measure antigen-antibody interactions by "real-time" observation. When the sample flows through the antigen-fixed sensor chip, a continuous event signal is generated, and the signal changes as the refractive index changes. When an analyte binds to a ligand, the difference in mass causes a corresponding change in refractive index that is proportional to the amount (mass) of bound antibody in the sample being tested.
TDIM was characterized by SPR to measure serum concentrations of infliximab, antibodies against tumor necrosis factor-alpha (anti-TNFa) and anti-infliximab antibodies. Some scientists have found that it can reduce mAbs interference when detecting low-affinity antibodies to therapeutic mAbs. SPR can directly detect and measure the value and content of serum antibodies in a short period, avoiding other cumbersome steps such as incubation, separation, and washing, while reducing the complexity of the data and the variability of factors. Besides, multiple drugs and their corresponding antibodies can be measured simultaneously. This method has been proven to have good sensitivity and has a higher profit than the ELISA kit.
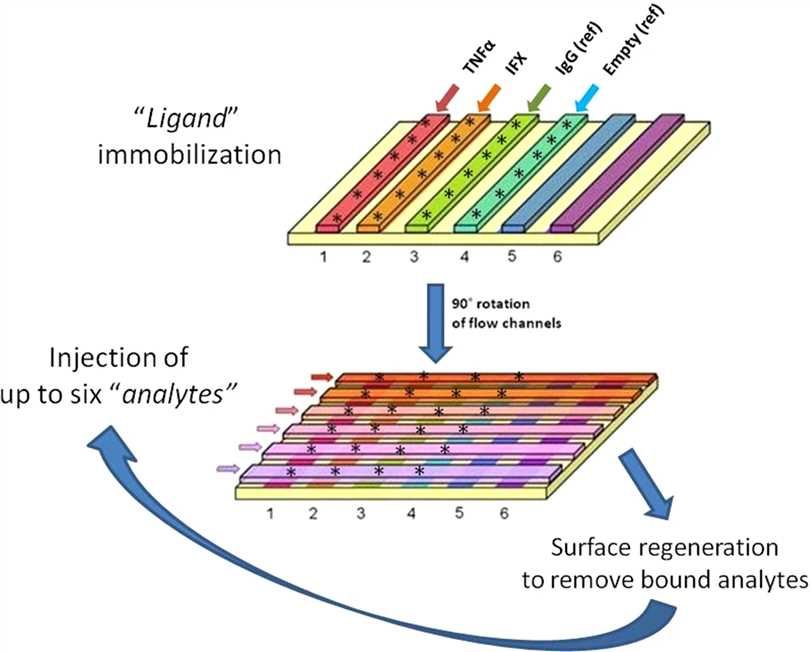 Fig.1 General scheme of the SPR-based assay for simultaneous determination of IFX and ATI concentrations in serum. (Beeg, 2019)
Fig.1 General scheme of the SPR-based assay for simultaneous determination of IFX and ATI concentrations in serum. (Beeg, 2019)
The real-time kinetic binding assay is suitable for the rapid analysis of data of various biotherapeutics and has broad application prospects. Creative Biolabs offers quality technical support of real-time kinetic binding assay and immunogenic detection. Please contact us as soon as possible and we are honored to serve you.
Reference
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.