Antibodies facilitate studies of lysine succinylation biology by identifying its substrates and molecular mechanisms. Creative Biolabs provides global customers with site-specific and pan anti-succinyllysine antibodies based on our excellent High-Affi™ technology. The antibodies are produced by immunizing animals with succinylated-lysine, or peptides with succinylated-lysine, conjugated to KLH and are purified by affinity chromatography. The product specifically recognizes proteins with succinylated lysine residues but not non-modified lysine residues or lysine residues similarly modified in structure.
Succinylation is a newly identified protein post-translational modification (PTM) which modifies the ε-amino group of lysine residues. Lysine succinylation is evolutionarily conserved and extensively distributes both in eukaryotic and eukaryotic cells. Succinylation is a dynamic and reversible process, but the regulatory enzymes remain largely unknown. Recent studies have shown that succinyl-CoA might be a cofactor for lysine succinylation. Several proteomic studies also reveal that SIRT5 is the major enzyme for lysine desuccinylation in mammalian cells. In addition, a known Sir2-like bacterial lysine deacetylase CobB also acts on desuccinylation function in prokaryotic cells. It is likely that lysine succinylation could overlap some regulatory roles relative to lysine acetylation under some physiological conditions. However, lysine succinylation induces larger changes in substantial structure than do lysine acetylation and methylation, as the succinyl group changes lysine’s charge from +1 to -1, from +1 to 0 and not at all, respectively. Meanwhile, different modified regions were also found between the lysine succinylation and lysine acetylation, which primarily exists at the C-terminal globular domains of histones, nearly all the lysine acetylation sites localize to the N-terminal domains. These suggest that succinylation may play a unique role in the regulation of protein function and activities. In recent years, scientists have paid more attention to study succinylation, revealing that succinylation occurs on various proteins such as histones, metabolic enzymes, transcription factors, and translocators. This modification implicates a range of disease states including metabolic disease such as diabetes, cancer, neurodegenerative diseases, and aging-related disorders.
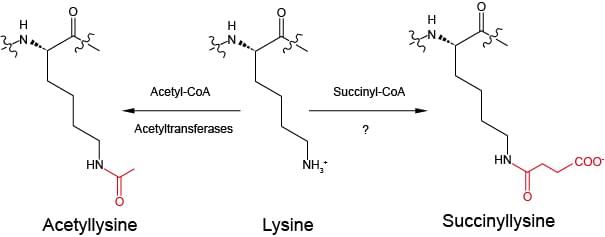 Fig. 1 Illustration of chemical structures of lysine, acetyllysine and succinyllysine residues (Zhang Z.H. et al. 2011)
Fig. 1 Illustration of chemical structures of lysine, acetyllysine and succinyllysine residues (Zhang Z.H. et al. 2011)
Creative Biolabs is an undoubted pioneer and experienced expert in antibody research and development field. Our scientists are committed to offering the most comprehensive antibody production services with feasible design and reliable results to meet our customers’ specific requirements and facilitate their research and project development.
In addition to the succinylation-specific antibody, Creative Biolabs also provides a comprehensive list of PTM-specific antibody production services of your choice.
Reference
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.