In recent years, monoclonal antibodies, showing promising therapeutic potential in disease areas such as cancer and autoimmune diseases, is a potential avenue to overcome hard-to-tackle disease areas. Monkey monoclonal antibodies have many similar characteristics to humans in certain research and therapeutic applications, which gives it a clear advantage over other laboratory animals. Related studies have demonstrated that the immunoglobulin genes of monkeys are quite close to those of humans. Creative Biolabs has the expertise and equipment to produce monoclonal monkey antibodies, as well as provide a comprehensive service to produce the right monoclonal monkey antibody for global clients. Our services include but are not limited to monoclonal monkey (NHP) antibody production, Native™ monkey (NHP) antibody discovery service, and humanization of monkey (NHP) antibodies.
 Fig 1. Schematic diagram of the study. (Gao, Y., et al., 2021)
Fig 1. Schematic diagram of the study. (Gao, Y., et al., 2021)
The selection of a suitable surrogate peptide lays the foundation for the establishment of an accurate and stable LC–MS/MS method. In this study, peptides in the trypsin mixture were identified using a nano-LC–Orbitrap fusion and the Mascot search engine. Fig 1. shows the total ion chromatogram (TIC) of all SHR-1222 peptides separated by the nano-LC system. After blasting against the Homo sapiens (for clinical trials) and Macaca fascicularis protein databases, non-unique peptides were excluded. To improve the efficiency of selecting the most suitable peptides, peptide mixtures were incubated at 60°C for 6 hours after quenching of the trypsin digest and prior to detection of the peptide mixture. The long time and high-temperature incubation helped to reduce the interference of unstable peptides.
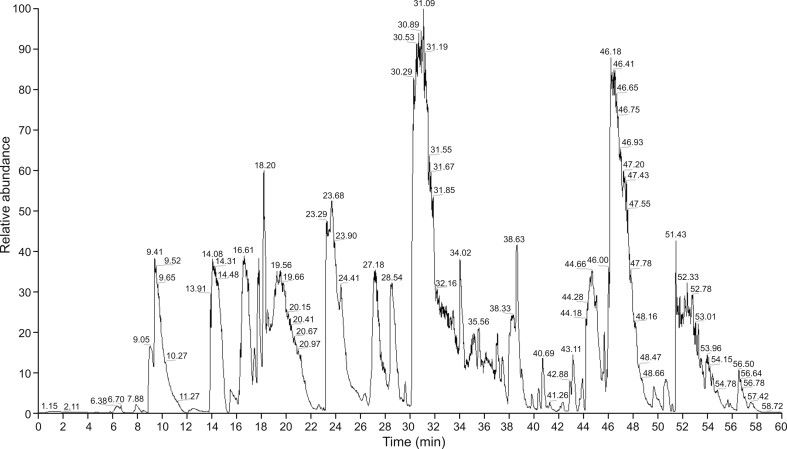 Fig 2. The total ion chromatogram (TIC) of all peptides of SHR-1222 digest separated by the nano-LC system. (Gao, Y., et al., 2021)
Fig 2. The total ion chromatogram (TIC) of all peptides of SHR-1222 digest separated by the nano-LC system. (Gao, Y., et al., 2021)
Optimization of LC–MS/MS methods includes adjustment of MS parameters, improvement of liquid chromatography conditions, simplification of pretreatment processes, and selection of the most suitable alternative peptides.
After the identification of candidate peptides, quantitative work was performed on the Agilent 6495 QqQ. It is worth noting that the sensitivity of QqQ MS is usually superior to that of high-resolution MS. Peptides often carry multiple charges in MS and optimizing them one by one is very time-consuming. Therefore, Skyline software was used in conjunction with Agilent MassHunter data acquisition software. Multiple scan windows were set up to simultaneously optimize the MS parameters of the candidate peptides by a single LC–MS/MS analysis (Figure 2). The peptide with LLIYYTSNR sequence in the antibody light chain was finally selected for quantitative analysis with a monitoring transition of m/z 571.8→803.4 and a collision energy of 19.0 V. In addition, the monitored conversion of LLIYYTSNR [15N,13C] was m/z 576.5→813 with a collision energy of 18.7 V.
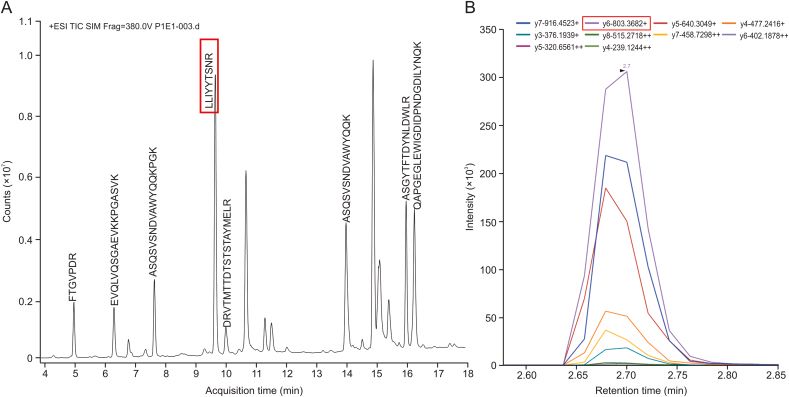 Fig 3. Optimization of MS parameters of the surrogate peptide. (A) The peptide LLIYYTSNR on the light chain of SHR-1222 showed the highest signal in full mass scan and (B) the transitions and collision energy of LLIYYTSNR were optimized using Skyline software. (Gao, Y., et al., 2021)
Fig 3. Optimization of MS parameters of the surrogate peptide. (A) The peptide LLIYYTSNR on the light chain of SHR-1222 showed the highest signal in full mass scan and (B) the transitions and collision energy of LLIYYTSNR were optimized using Skyline software. (Gao, Y., et al., 2021)
The accuracy and precision of the LC–MS/MS method were evaluated by 3 different batches of analytical tests performed by 2 analysts for at least 2 days, and the results of the batch analysis were considered to be acceptable. The results of the LC–MS/MS method validation showed that the matrix effects of the hemolyzed samples did not significantly affect the accuracy and precision of the quantification. Notably, the accuracy and precision of QC samples were good, indicating that both extraction recovery and digestion efficiency could meet the requirements of method validation. The results showed that the target mAb was stable in serum samples for more than 5.0 h at room temperature,191 d at -70 °C, and 5 freeze-thaw cycles.
The validated method was applied to the TK assessment of SHR-1222 (60 mg/kg). The result showed drug exposure ratios of 1.21-1.45 between the last administration (7th) and the first, indicating a slight accumulation of SHR-1222 in cynomolgus monkeys after 12 weeks of subcutaneous administration (60 mg/kg). These results were consistent with the previous study by the MSD–ECL method.
For some anomalous data during the experiment, the investigators identified possible causes through an in-depth investigation of immunogenicity, ADA interference, and other factors. The results showed that the three abnormal samples had very high anti-SHR-1222 ADA levels with titers ≥16, whereas the other samples had ADA titers of only about 2. Besides, it is hypothesized that high ADA levels could accelerate the clearance of SHR1222 in vivo, characterized by reduced drug exposure rather than ADA interference with detection.
Compared with the conventional MSD–ECL, LC–MS/MS has a higher specificity and dynamic range. At the same time, the LC–MS/MS is simpler and time-saving to meet the requirements of high-throughput TK analysis. Low sensitivity is the main drawback of LC–MS/MS for the quantification of large-molecule drugs. On the one hand, it is due to the high homogeneity of plasma proteins in the matrix, though the multiple reaction monitoring mode can improve the detection sensitivity to some extent. On the other hand, peptides tend to carry multiple charges, leading to reduced ionization efficiency and dispersion signals. Although low-flux LC–MS (e.g., nano-LC–MS) and hybrid LC/MS methods have been developed to improve detection sensitivity, they result in low reproducibility and throughput. Therefore, more technological innovations are needed and a balance between sensitivity and throughput is needed according to the analytical requirements. The present work concluded that the sensitivity of our LC–MS/MS method can meet the quantitative requirements due to the high concentration of in vivo monoclonal antibody detection.
Reference
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.