Kinases are essential mediators of numerous cellular signaling pathways and are intimately involved in a wide range of disease processes. Many small molecule and antibody inhibitors that target kinases have received clinical approval, but these drugs still face some obstacles. Constraint peptides, on the other hand, offer many advantages that can bridge these gaps. For example, constraint peptides can provide a specific binding surface area, allowing them to bind to shallow protein surfaces. Chemical restrictions within the peptide sequence can improve target affinity and target selectivity, cell permeability, and protein hydrolysis resistance. Thus, constraint peptides are an alternative strategy to kinase inhibitors. Given the rich activity and function of constrained peptides, Creative Biolabs targeted constrained peptide library construction provides a one-stop scientific platform.
Small molecule inhibitors frequently contain ATP-binding sites in order to target kinases, but achieving selectivity remains challenging. Constrained peptide-small molecule binding can partially address this issue. Constrained peptides can confer improved selectivity and affinity for small molecule inhibitors, and this approach has been applied in the development of inhibitors for a variety of kinases. However, the method still has some drawbacks. Due to the large overall size of the constrained peptide-small molecule, the therapeutic potential of the current target intracellular kinase structural domain may be limited.
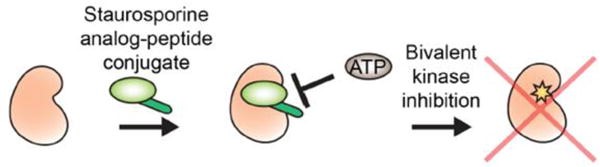 Fig 1. Peptide-molecule conjugates targeting the ATP-binding site. (Hanold, L. E., et al., 2017)
Fig 1. Peptide-molecule conjugates targeting the ATP-binding site. (Hanold, L. E., et al., 2017)
Peptides targeting the ligands or the ligand-binding domain of enzyme-linked receptors can be designed to act as modulators of kinase activity. This enables the regulation of signal transduction cascades that contribute to various cellular processes. For example, among enzyme-linked receptors, receptor tyrosine kinases have an important role in cell growth and motility. This makes it a target for the development of anti-proliferative, anti-metastatic, and anti-angiogenic compounds. Constrained peptides can target ligand-induced receptor tyrosine kinase activation by blocking the receptor-binding surface of the ligand or by closing the ligand-binding site of the receptor.
One of the main advantages of targeting ligands or ligand-binding domains is that cell permeability of the targeting agent is not required. This is because the target is extracellular. In addition, these constrained peptides do not target highly conserved kinase active sites, and there is greater diversity among ligand binding sites, which may limit the off-target effects typically observed with competitive inhibitors of ATP. However, the peptide must outcompete the ligand, which may be overexpressed in certain disease states. Furthermore, this targeting method is mainly restricted to the receptor tyrosine kinase subfamily, as the vast majority of kinases lack an extracellular ligand binding domain.
Allosteric mechanisms are also important in regulating kinase activity. Dimerization of the receptor tyrosine kinase contributes to the activation of the kinase structural domain and the subsequent tyrosine phosphorylation events. Therefore, the use of constrained peptides to disrupt dimerization or allosteric activation is a promising strategy to regulate kinase activity. A number of constrained peptides with related functions have been developed. Taking the EGFR signaling pathway as an example, the constrained peptide can disrupt receptor dimerization by targeting the EGFR dimerization arm, thereby blocking the EGFR signaling pathway in cancer.
The use of constrained peptides that target kinase variable sites may limit off-target effects because they do not need to target highly conserved active sites to modulate kinase activity. Moreover, these peptides do not need to compete with ligand binding. Disrupting dimer interactions to modulate kinase activity may, however, be influenced by the protein's expression level, particularly in the presence of preformed dimers.
Reference
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.