T & B Cell based Binding Assay Service
Background What We Can Offer Workflow Why Choose Us FAQs Customer Review Related Services Contact Us
To better understand vaccines or drugs' immune response to pathogens, it is necessary to investigate T and B cell epitopes to a specific antigenic peptide. With advanced technology and excellent in-house expertise, Creative Biolabs provides T and B cell-based binding assays for cancer epitope analysis.
T and B-cell Binding Epitope
T cell receptor (TCR) and B cell receptor (BCR) is responsible for pathogens recognition. The specific portions of antigens that bind to the receptor are named antigen determinants or epitopes. An antigen can have one or more epitopes. Typically, antibodies bind exposed solvent parts of the antigen with about five amino acids or sugars in size. TCRs recognize the cancer epitopes attached to major histocompatibility complex (MHC) molecules exhibited on the antigen-presenting cells (APCs), roughly 8 to 17 amino acid residues, including MHC I-presented epitopes and MHC II-presented epitopes.
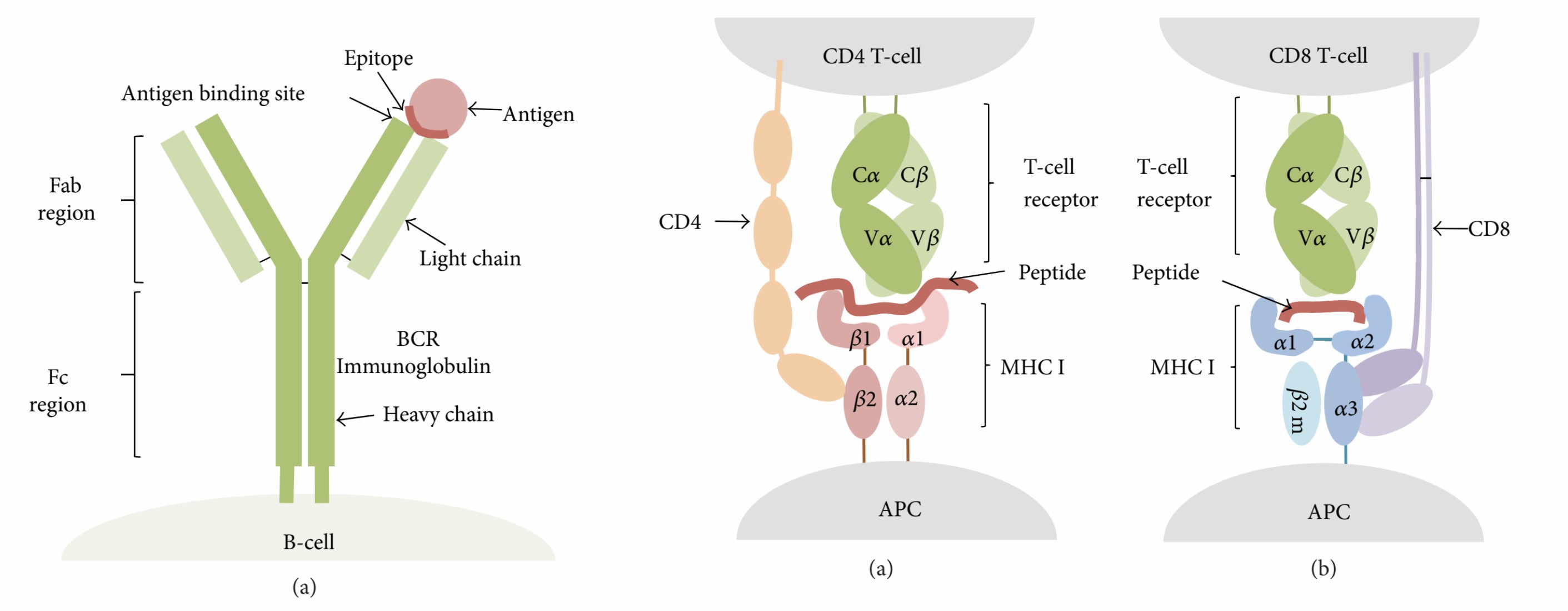 Fig.1 T and B cell-based epitope recognition.1
Fig.1 T and B cell-based epitope recognition.1
A Hybrid Approach: Combining In Silico and In Vitro Analysis
-
In Silico Epitope Prediction
A high-throughput method that can rapidly identify potential epitopes within an antigen sequence.
-
Kinetics Data
In quantitative binding assays, the binding strength of the receptor to the antigen peptide (ligand) is precisely measured. We provide the following key kinetic data:
-
Dissociation constant (KD): The equilibrium dissociation constant, KD, describes the binding affinity between a specific antibody and antigen. It is commonly calculated as a ratio of the off-rate to the on-rate (KD=koff/kon).
-
On-rate (kon): The association rate of the antibody, which describes how quickly the antibody binds to its antigen.
-
Off-rate (koff): The disassociation rate of an antibody, which describes how quickly the antibody dissociates from its antigen.
-
Affinity constant (KA): When the antibody-antigen complex reaches equilibrium, the affinity constant is the inverse of the dissociation constant (KA=1/KD). A smaller KD value corresponds to a stronger affinity of the antibody for its target.
-
Qualitative Binding
We also provide qualitative analysis, which can determine the antibody response levels to different epitopes and detect T cell expansion using techniques such as a multimer/tetramer assay.
Please contact us today to discuss how our cutting-edge T and B cell-based binding assays and epitope prediction services can illuminate your path to discovery and unlock the full potential of your therapeutic candidates.
Workflow
Our service is designed as a clear and intuitive workflow that can be easily integrated into your project timeline.
Required Starting Materials:
-
Antigen Sequence Information: The amino acid sequence of your target protein.
-
Therapeutic Candidates: Your lead antibody or vaccine candidate.
-
Target Cell Line Information: Details on the specific cell line or primary cells expressing your target antigen.
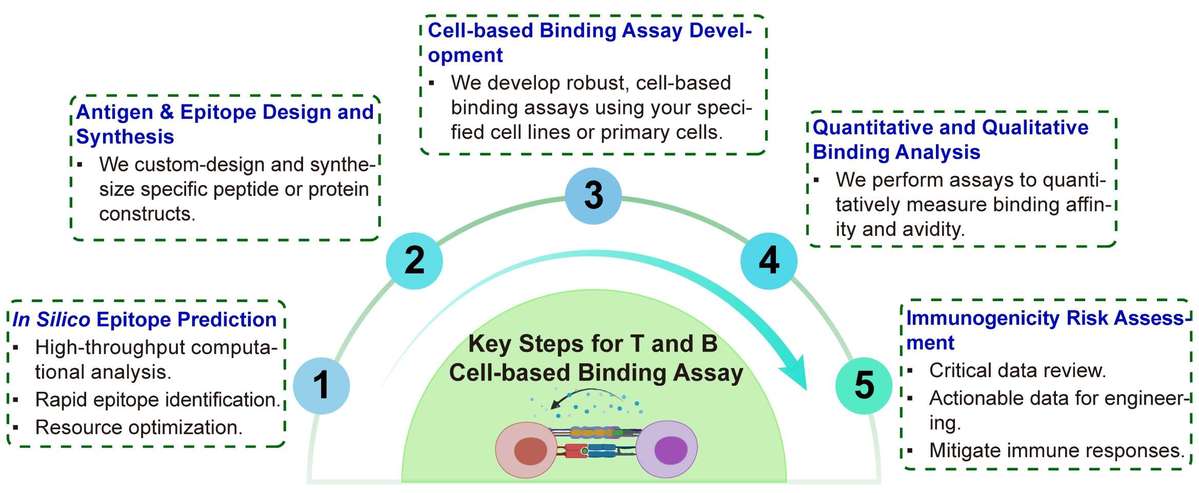
Final Deliverables:
-
Comprehensive Project Report: A detailed report containing all raw data, analysis, and interpretation of the results.
-
Binding Kinetics Data: Quantitative values for KD, kon, and koff that describe the binding affinity and kinetics of your therapeutic candidate.
-
Immunogenicity Risk Profile: A clear summary of potential immunogenic epitopes and recommendations for risk mitigation.
Why Choose Us?
Choosing Creative Biolabs means partnering with a leader in immuno-oncology, combining deep scientific expertise with state-of-the-art technology. Our hybrid in silico and in vitro platform offers a competitive advantage, enabling high-throughput screening and validation in a physiologically relevant context. This approach is supported by extensive published data and a track record of success in helping clients bring projects to fruition. We provide comprehensive data analysis and expert interpretation, ensuring reliable and actionable insights for your research. Partner with us to accelerate your drug discovery and development in the dynamic field of immuno-oncology.
FAQs
Q1: Why should I choose a cell-based assay over a traditional one like SPR or ELISA?
A1: It accounts for the complexities of antigen presentation and recognition by living T and B cells, offering a more accurate prediction of in vivo binding and potential immunogenicity, which is essential for drug and vaccine safety.
Q2: How accurate are your in silico epitope predictions?
A2: Computational prediction is a powerful screening tool, but it's not a standalone solution. We use it to identify the most likely epitope candidates, which are then rigorously validated with our in vitro assays.
Q3: Can your services handle high-throughput screening for a large library of candidates?
A3: Absolutely. Our platform is designed for scalability and high-throughput screening. We can efficiently handle large-scale projects, allowing you to screen entire libraries of therapeutic candidates and identify those with the most promising binding characteristics in a fraction of the time.
Customer Review
-
Early-Stage De-risking
Using Creative Biolabs' T and B cell-based binding assays in our research has significantly improved our ability to de-risk our candidates early on, saving us both time and money in the long run. - Dr. L. R. Bro***
-
Precision and Informed Decision-Making
The detailed binding kinetics data we received from Creative Biolabs were instrumental in our drug development program. The precision of the KD and kinetic data helped us make informed decisions about our lead candidate's mechanism of action. - Dr. E. P. Wil***
Related Services
-
Binding interaction can be measured using various advanced techniques regarding your sample and requirements.
-
Combined binding assay with multiple 3D structure methods to determine epitope location and orientation.
Contact Us
At Creative Biolabs, we are dedicated to providing innovative solutions that help our clients navigate the complex world of drug and vaccine development. Our T and B cell-based binding assays, powered by our hybrid in silico and in vitro platform, are designed to accelerate your timelines, de-risk your projects, and save your research time and inputs.
For detailed information and to discuss your project requirements with one of our specialists, please do not hesitate to reach out.
Reference
-
Sanchez-Trincado, Jose L., Marta Gomez-Perosanz, and Pedro A. Reche. "Fundamentals and methods for T‐and B‐cell epitope prediction." Journal of immunology research 2017.1 (2017): 2680160. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.1155/2017/2680160


 Fig.1 T and B cell-based epitope recognition.1
Fig.1 T and B cell-based epitope recognition.1

