Creative Biolabs provides high diversity Hi-Affi™ phage display α2p8 library for isolation and identification of the high affinity target binders. Our scientists are enthused in providing our customers with the most appropriate project design for each individual requirement at reasonable budget.
α2p8 is a well-defined 38 amino acids miniprotein derived from the N-terminal region of a human protein mature T-cell proliferation 1 (p8MTCP1, a small 8 kDa protein encoded by the human oncogene MTCP1). Based on the natural structure of p8MTCP1, which consisting of three α helices, α2p8 is composed of two α helices linked by a short loop to form a α-helical hairpin structure that represents the two N-terminal helices of the p8MTCP1 and keeps its native coiled-coil structure, while the third helix which defined as an antiparallel α-helical motif in p8MTCP1 has been removed. Furthermore, the structure of α2p8 is stabilized by two disulfide bridges between the two α helices and several hydrophobic interactions at the helix interface. Comparing with p8MTCP1, the α2p8 is more stable and permissive for sequence modification; most of solvent-exposed surface of α2p8 is permitted for further engineering a protein recognition surface either by the transfer of suitable functional sites or by sequence randomization and functional selection. In addition, α2p8 is easily amenable to synthetic chemistry and biological expression system. In this way, α2p8 may potentially represent a stable and versatile scaffold or the minimal mimic of a two stranded α-helical scaffold to be modified and displayed as scaffold libraries for the discovery of novel binding ability or exogenous functional site.
Creative Biolabs is able to generate the α2p8 scaffold libraries through our proprietary Hi-Affi™ phage display platform. Through phage display technology, the gene of interest is fused to bacteriophage coat proteins and therefore displayed on the phage surface; the pool of the phages is then mixed with an immobilized target to isolate specific binders. Devised to expand the range of applications of specific protein scaffolds, our scientists have combined the trimer codon technology and NNK method in the development of Hi-Affi™ platform. In this way, the platform is more suitable for sorting and isolating the high affinity protein or peptide targets and able to offer 100% precise mutant library construction with over 1010 diversity.
Creative Biolabs has long-term extensive experience in the field of scaffold library construction. Our technical experts are committed to offering exquisite service and first-class technology for customers to assist their research and boost project progression.
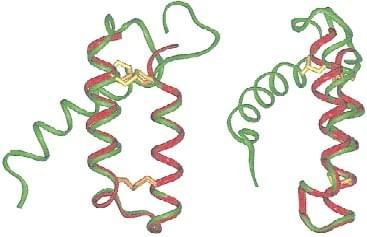 Fig. 1 Ribbon diagram of the average NMR structure of α2p8 (red) superimposed to the average NMR structure of p8MTCP1 (green) from residue 3 to residue 34. (Barthe et al. 2000)
Fig. 1 Ribbon diagram of the average NMR structure of α2p8 (red) superimposed to the average NMR structure of p8MTCP1 (green) from residue 3 to residue 34. (Barthe et al. 2000)
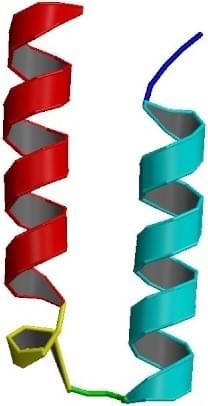 Fig. 2 NMR structure of the α-helical hairpin of p8MTCP1. (PDB ID: 1EI0 )
Fig. 2 NMR structure of the α-helical hairpin of p8MTCP1. (PDB ID: 1EI0 )
Reference
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.