Scaffold proteins are defined as organizing platforms that link at least two protein partners. The ability of scaffold proteins to selectively recruit signaling proteins provides a tightly regulated and dynamic regulatory mechanism for cellular signaling. Covalent scaffolds are multidomain scaffold proteins that are engineered by genetic conjugation of scaffold domains via linkers. The significance of covalent scaffolds in synthetic signaling pathways, metabolic engineering, and liquid-liquid phase separation, among other applications. In view of the various functions of scaffold proteins, Creative Biolabs offers advanced engineered protein scaffold library construction services to meet the research needs of investigators.
Currently, the role of scaffold proteins in regulating the flux of information within signaling pathways is being investigated primarily using synthetic variants of natural scaffolds. This approach has been utilized extensively for well-characterized scaffold proteins of various mitogen-activated protein kinase (MAPK) pathways. The following results can be derived from the available literature. Using conjugated multidomain scaffolds to generate a synthetic MAPK signaling pathway demonstrates a higher-order function of scaffold proteins as centers for signal processing. As the target of a feedback loop, scaffold proteins alter the signaling amplitude and timing. Furthermore, the reorganization of scaffold structural domains suggests that scaffolds are modular and flexible tissue centers whose responses can be modulated by simple changes or rearrangements in the recruitment of structural domains. Of course, in addition to MAPK scaffolds and synthetic variants, other synthetic modules were designed to study signaling pathways, such as the actin regulatory switch N-WASP.
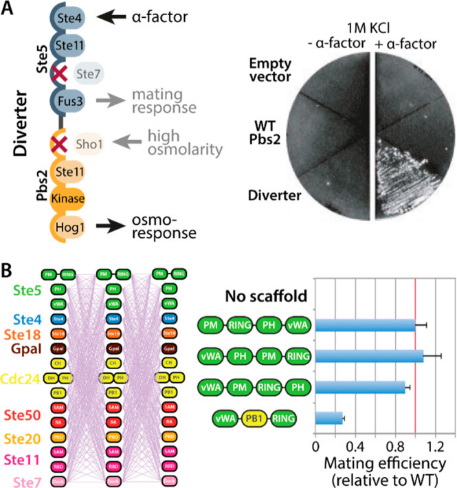 Fig 1. Plasticity in the MAPK pathway by modular recombination of MAPK scaffold domains. (Lemmens, L. J. M., et al., 2020)
Fig 1. Plasticity in the MAPK pathway by modular recombination of MAPK scaffold domains. (Lemmens, L. J. M., et al., 2020)
Synthetic scaffolds can be used to generate artificial metabolic pathways. In synthetic enzyme complexes, enzymes with different activity levels can lead to suboptimal pathway fluxes through the accumulation of intermediates. In this process, optimization of the enzyme stoichiometry can overcome this imbalance in flux. This regulation and control of stoichiometry can be achieved through the synthetic scaffold. With the advancement of related research, the field of metabolic engineering has led to the understanding that reliance on enzymatic pathway assembly on conjugated multidomain scaffold proteins has generated a variety of synthetic metabolites. The reorganization of these structural domains has allowed the optimization of metabolic fluxes and the introduction of new biosynthetic pathways, thus greatly expanding the functional applications of synthetic protein complexes.
Cell signaling is tightly regulated in time and space through various mechanisms, such as classical organelles, scaffolding proteins, and membraneless organelles. These membraneless organelles are biomolecular condensates that are capable of concentrating proteins and nucleic acids. In studies targeting biomolecular condensates, scientists have found that multidomain proteins allow precise control of valency and monovalent affinity and are ideal models for research. The findings of many scientists suggest that the sharp transitions observed in liquid-liquid phase separation are driven by multivalent interactions between the scaffolds and their respective ligands and in addition, provide a model for the subsequent recruitment of low valency clients in these droplets. Thus, the distribution of cell signaling molecules in these phase separation systems provides a regulatory mechanism that produces nonlinearity in the signaling pathway.
Reference
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.