Creative Biolabs has long-term devoted to the development and application of scaffold proteins. Based on the thermostable HEAT-like repeats, now we are able to construct a new family of artificial alpha-helicoidal repeat protein (αRep) to meet the specific needs of our clients. Based on our powerful Hi-Affi™ platform, we can offer the library with high affinity and diversity.
Introduction of Repeat Protein and HEAT (αRep)
Repeat proteins consist of tandem arrays of a small structural motif. The modular organization and regular architecture make them attractive models for biological research and applications. Compared with the typical globular proteins, repeat proteins are extended structures that have a large ratio of surface area to volume. Upon the unique structure, repeat proteins have varies of specific functions, such as the ability to mediate protein-protein interactions, organize the multiple proteins into functional complexes and bind to different ligands according to the different sets of repeats. As a member of repeat protein family, HEAT always shows 3-36 repeats for natural proteins, the number of amino acids in each repeat is 37-47 and the structural motif of a repeat is two α-helices (A & B). HEAT repeats are versatile binding modules that not only play an important role in protein-protein interactions but also bind to the nucleic acids.
The Difficulty and Conception of HEAT (αRep) Library
Although very common, HEAT repeat proteins have not been widely used as a scaffold protein because of their long and irregular repeats. With the foundation of the known structure of a thermostable HEAT repeat subfamily, the well-defined consensus sequence and the adapted N-caps and C-caps for the stable HEAT repeats have been identified. Now Creative Biolabs can build the library called αRep based on the consensus sequence of HEAT-like repeat proteins. The biophysical properties of proteins selected from the library are characterized by the different numbers of repeats and the different combinations of side chains in hypervariable positions.
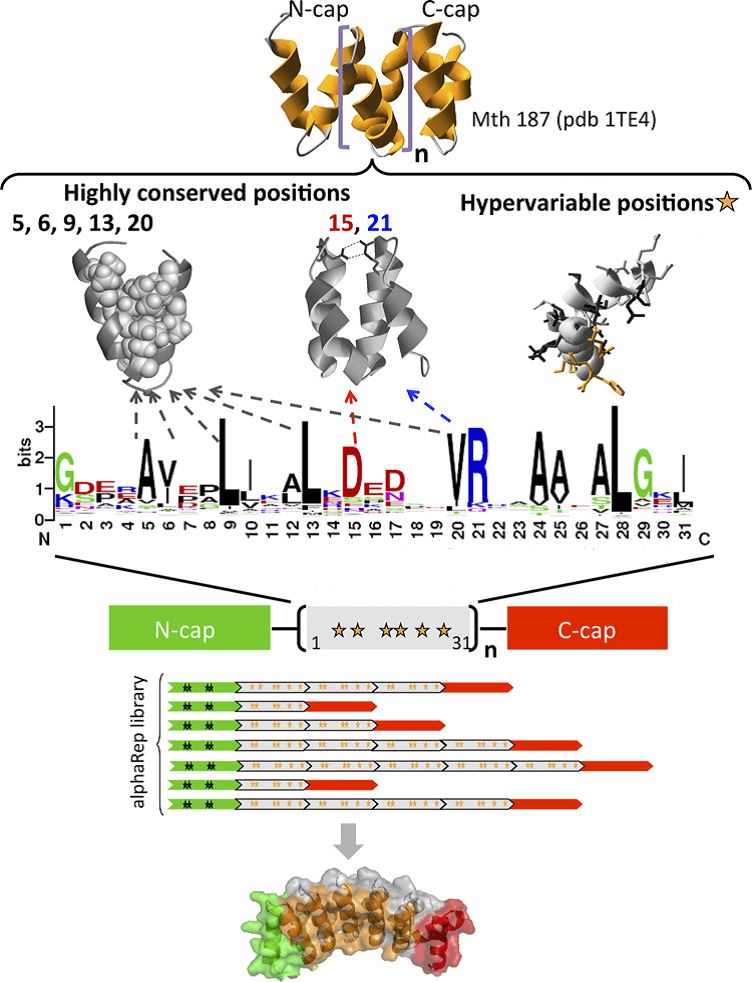 Fig.1 Construction of the αRep library using a consensus design approach. (Valeriolepiniec, M.; et al. 2015)
Fig.1 Construction of the αRep library using a consensus design approach. (Valeriolepiniec, M.; et al. 2015)
Construction of HEAT (αRep) Library
In αRep library construction, Mth187 is used to be a structure template as this protein is the first known structure of stable HEAT-like repeats. The 31 amino acids αRep motif is coded and the degeneration can correspond to the several hypervariable positions as most positions of the sequence are highly conserved. After a series of polymerase chain reactions, digestion, ligation, and transformation, we can obtain a αRep library with 1.7×109 clones which are different in the number of inserted motifs and the randomized positions in each motif. As a result, proteins from this library show great advantages, such as well expressed, high solubility, thermostable, and expected fold.
Based on our extensive experience and advanced platform, Creative Biolabs has the ability to provide more than 50 kinds of scaffold libraries construction service for our customers all over the world. We are therefore confident in offering the specific service to free up your time for core work and project. If you are interested in our service, please do not hesitate to inquire us for more details.
Reference
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.