- Home
- UTC Development
- Antibody-Biopolymer Conjugate (ABC) Development
Antibody-Biopolymer Conjugate (ABC) Development Service
Currently, the therapies for retinal disease mainly depend on intravitreal administration and require frequent injections due to short duration of action after each injection, such as retinal diseases. Fortunately, a new therapy method referred to antibody biopolymer conjugate (ABC) has been developed to present improved efficacy and excellent safety. Several ABCs are developed and evaluated actively in different clinical trial phases presently. With years of experience in antibody development and antibody-related bioconjugation such as antibody-drug conjugates (ADCs), Creative Biolabs now offers one-stop ABC design and construction services. Our innovative linker technologies and effective bioconjugation platform will promote your drug discovery and development.
Introduction of Biopolymer
Biopolymers are natural polymers synthesized by living organisms. Biopolymers mainly include three classes, polynucleotides (such as the nucleic acids DNA and RNA), polypeptides (proteins), and polysaccharides (polymeric carbohydrates). These consist of long chains formed from repeating, covalently bonded monomers, such as nucleotides, amino acids, or monosaccharides. Biopolymers can be used in biomedical engineering to mimic body parts to sustain normal body functions. Due to their biocompatibility, biopolymers are widely used in tissue engineering, medical devices, and pharmaceutical industries. In addition, a variety of biopolymers can be used for regenerative medicine, drug delivery, and overall medical applications. They are rendered unique characteristics like wound healing, catalysis of bio-activity, and non-toxicity. Many biopolymers are normally better with bodily integration as they also possess more complex structures, similar to the human body.
 Fig.1 Biopolymers for medical applications. (Ruso, 2017)
Fig.1 Biopolymers for medical applications. (Ruso, 2017)
Antibody Biopolymer Conjugates
The ABC technology is a novel approach to produce potent and effective drugs designed to have enhanced durability and subsequent clinical effect. KSI-301 is the first ABC composed of a recombinant, full-length, humanized anti-VEGF IgG1 monoclonal antibody conjugated to a high molecular weight phosphorylcholine-based biopolymer. It is designed to provide extended ocular half-life and increased tissue bioavailability. The conjugate can bind to human VEGF-A with a high binding affinity, just like currently available anti-VEGF therapies. Simply, the optically clear phosphorylcholine biopolymer is covalently bound through site-specific conjugation to the antibody, which results in an intentionally high molecular weight of 950 kDa. Preclinical pharmacokinetic studies demonstrated that KSI-301 has extended ocular half-life (10-12 days) compared to ranibizumab (3-4 days) or aflibercept (4-5 days). Besides, KSI-301 can be cleared rapidly when it leaves the eye. Importantly, KSI-301 can be injected intravitreally using typical clinical procedures and equipment. It is currently in clinical testing for the treatment of retinal vascular diseases.
Features of ABC
- Improved bioavailability
- Improved biocompatibility
- Improved stability
- Fast systemic clearing
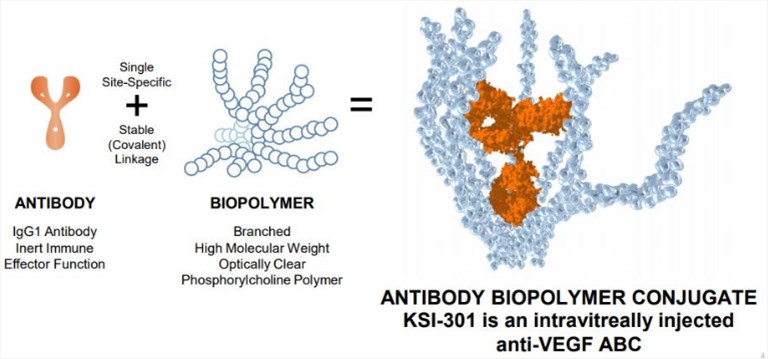 Fig.2 A schematic description of KSI-301. (Patel, 2019)
Fig.2 A schematic description of KSI-301. (Patel, 2019)
Comprehensive ABC Development Service at Creative Biolabs
Creative Biolabs’ first-class services utilize various advanced technology platforms to produce therapeutic antibody candidates. Our Antibody Design and Conjugation services will achieve effective antibody-biopolymers conjugation. We will choose optimal conjugation strategy to obtain the best conjugates. Furthermore, we also provide a comprehensive set of ABC analysis services. Relying on our extensive experience and advanced equipment, Creative Biolabs offers high-quality ABC preparation service in accordance with your specific needs.
Contact us today for a free consultation with the scientific team and discover how Creative Biolabs can be a valuable resource and partner for your organization.
References
- Ruso, J. M.; Messina, P. V. (Eds.). Biopolymers for Medical Applications. CRC Press. 2017.
- Patel, S. S., et al. Phase 1 first-in-human study of KSI-301: a novel anti-VEGF antibody biopolymer conjugate with extended durability. Investigative Ophthalmology & Visual Science. 2019, 60(9), 3670-3670.
For Research Use Only. NOT FOR CLINICAL USE.

Online Inquiry
Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
