- Home
- Resources
- Knowledge Center
- Protocols
- Analysis of ADCs by SEC-Native MS/IM-MS
Analysis of ADCs by SEC-Native MS/IM-MS
Native MS' hyphenation to ion mobility spectrometry (IM-MS) allows differentiation of gas-phase ions based on their rotationally averaged collision cross section, providing an additional dimension of conformational characterization of ADCs. However, sample preparation (desalting) is still mostly manual, labor-intensive, and time-consuming prior to native IM-MS analysis. An interesting technique, size exclusion chromatography (SEC), can perform online buffer exchange in an automated way prior to native MS/IM-MS analysis, which not only allows online buffer exchange within a few minutes but also provides separation, identification, and quantification of high/low molecular weight species.
This article takes deglycosylated ADCs as an example to briefly introduce how to characterize ADC by online SEC-native MS/IM-MS to perform structural analysis, aiming to help researchers understand the most cutting-edge methods related to ADC characterization.
Disclaimer
This procedure is only a guideline. Please note that Creative Biolabs is unable to guarantee experimental results if it is operated by the customer.
Online SEC-Native MS Analysis
Material:
100 mM AcONH4
2 g/L cesium iodide solution
Ultra-performance liquid chromatography (UPLC)
UPLC column (see Note 1)
Procedure:
1. Perform MS calibration with 8 μL of cesium iodide solution.
2. Setup the tune page parameters:
- The MS is operated in sensitive mode with a capillary voltage of 3.0 kV.
- The sample cone and pressure in the interface region were set to 180 V for lysine conjugate and 120 V for cysteine conjugate (see Note 2) and 6 mbar, respectively.
- Source and desolvation temperatures were set to 100 and 450 °C, respectively.
- Desolvation and cone gas flows were set at 750 and 60 L/h, respectively.
3. Set up the MS file parameters:
- Start and end times were set to 0 and 12 minutes, respectively.
- Polarity was set to positive.
- Analyzer mode was set to sensitivity.
- The dynamic range was set to normal.
- Sensitivity was set to normal.
- Low and high mass limits were set to 1000 Da and 10,000 Da, respectively.
- The scan time was set to 1.5 s (continuum).
- There should be no trap or transfer of CE.
- Events for flow state are created to direct salts to waste: 0 min flow state directed to waste, 4 min flow state directed to LC, 9 min flow state directed to waste.
- The injected volume should be between 1 and 10 μL.
4. Equilibrate the LC system with ten-column volumes of mobile phase.
5. ADC sample was injected (see Note 3), and then the elution process was conducted in an isocratic mode with a flow rate gradient consisting of the following three phases:
- A flow rate of 0.25 mL/min for 4 minutes
- A flow rate of 0.1 mL/min for 5.5 minutes
- A flow rate of 0.25 mL/min for 2.5 minutes
6. The average DAR was calculated based on the relative peak intensities measured from the raw mass spectra by using the following equation. (see Note 4).
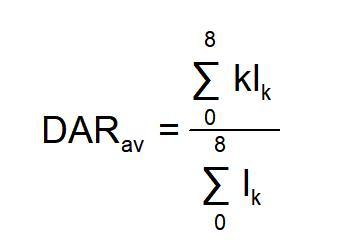
Where k is the number of drugs and Ik is the relative peak intensity of DARk.
Note:
1. Other columns are available for the SEC-native MS experiment. It is recommended to use a shorter column dimension for a unique fast desalting goal and a longer column dimension for a separation goal.
2. A high voltage (180 V) increases the transmission of high m/z ions along with more efficient desolvation. However, high Vc values also favor the dissociation of noncovalent assemblies, especially for cysteine-linked ADCs. It is usually more appropriate to use an intermediate Vc value of 100–120 V.
3. SEC-native MS can automatically desalt online without ADC sample preparation (desalting) with a micro concentrator.
4. SEC-native MS coupling provides higher MS spectral resolution and mass accuracy due to the more efficient desalting afforded by SEC compared to manual desalting.
SEC-Native IM-MS Analysis
Material:
Data analysis software
10 mM AcONH4
IM calibrants with concanavalin A, alcohol dehydrogenase, and pyruvate kinase at concentrations between 30 and 40 μM (see Note 4).
Procedure:
1. Adjust the following parameters in the data analysis software window:
- Select positive ion mode.
- Set the m/z range from 1,000 to 10,000.
- The sampling cone was set to 80 V.
- Backing pressure was set to 6 mbar.
- The source offset was set to 5 V.
- Source temperature and desolvation temperature were set to 100 °C and 450 °C, respectively.
- Trap and transfer collision energies were set to 4 V and 5 V, respectively.
- Trap gas was set to 5 mL/min.
2. Set the following parameters in the ion mobility cell: The N2 and He flow rate are 45 mL/min and 130 mL/min, respectively. Adjust the IM wave height to 40 V and set the IM wave velocity to 923 m/s.
3. Set up the MS file parameters: The parameter settings in this step are the same as in step 3 of the previous procedure for online SEC-native MS analysis.
4. Load the SEC column with 1 to 10 μL of each individual solution of IM calibrants.
5. Take three measurements of the IM chromatogram for each individual calibrant using the same instrumental conditions as in the previous ADC SEC-native MS analysis.
6. Determine the drift time associated with each peak in the calibrant solutions based on the chromatogram (refer to Note 5).
7. Utilize a series of provided equations in the article to convert the drift time of different DAR populations in your ADC sample into collision cross-section (CCS) values.
Note:
5. The choice of ion mobility calibrants for the analyzed mAb and ADC depends on their mass and charge state. In this experimental, calibrants ranging from 100 kDa to 230 kDa and with positive charge states between 15+ and 35+, generating ADCs are proteins around 150 kDa with 20+ to 28+ positive charges.
6. For concanavalin A, take the drift time of the 19+, 20+, and 21+ charge states. For ADH, take the 24+, 25+, and 26+ charge states. For PK take the 32+, 33+, and 34+ charge states.
For Research Use Only. NOT FOR CLINICAL USE.

Online Inquiry
Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
