Clinical Trials Analysis of TROP-2-Directed ADC for NSCLC
Trophoblastic Cell Surface Antigen 2 (TROP-2)
TROP-2 is expressed in normal tissues and in many epithelial tumors, including breast, cervix, colorectal, esophagus, gastric and lung cancer, and mediates several intracellular signaling pathways, including PTEN/PIK3CA/Akt, MAPK/ERK, JAK/STAT, ErbB, TGFβ and WNT/βcatenin. In non-small cell lung cancer (NSCLC), high TROP-2 expression has been observed in 64% of adenocarcinomas and 75% of squamous cell carcinomas (SqCCs). TROP-2 overexpression has been reported to be associated with poor prognosis in NSCLC. Therefore, there is an urgent need to understand the relationship between TROP-2 overexpression and poor prognosis in NSCLC to accelerate the development of next-generation novel TROP-2 directed anticancer therapy.
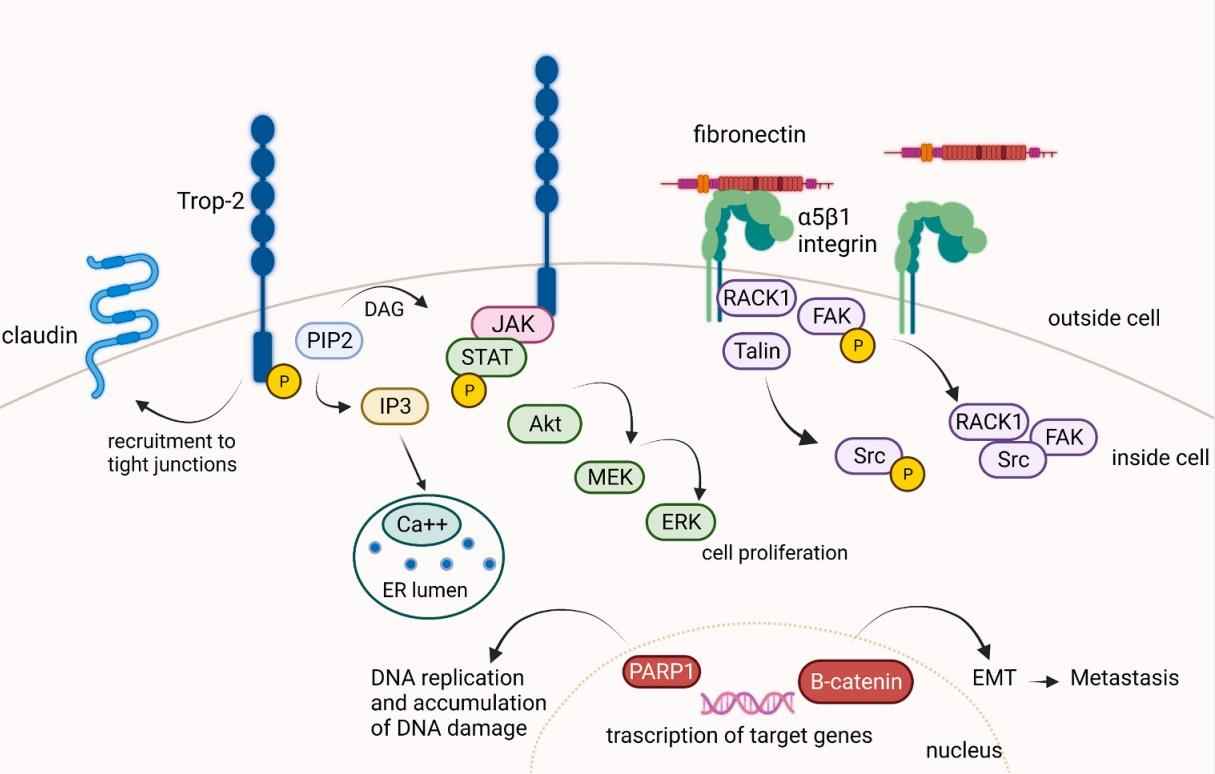 Fig. 1. TROP-2 mediates several intracellular signaling pathways. (Parisi C, et al., 2018)
Fig. 1. TROP-2 mediates several intracellular signaling pathways. (Parisi C, et al., 2018)
Creative Biolabs has extensive experience in the field of developing ADCs and provides ADC development for NSCLC and ADC development services targeting TROP2.
In the study, scientists performed a literature search of the clinical trials about TROP-2-directed ADCs in NSCLC referenced in the Pubmed.gov database, Cochrane Library database, and clinicaltrial.gov database.
ADCs targeting TROP-2, Sacituzumab Govitecan and Datopotamab Deruxteca, in NSCLC were conducted system analysis.
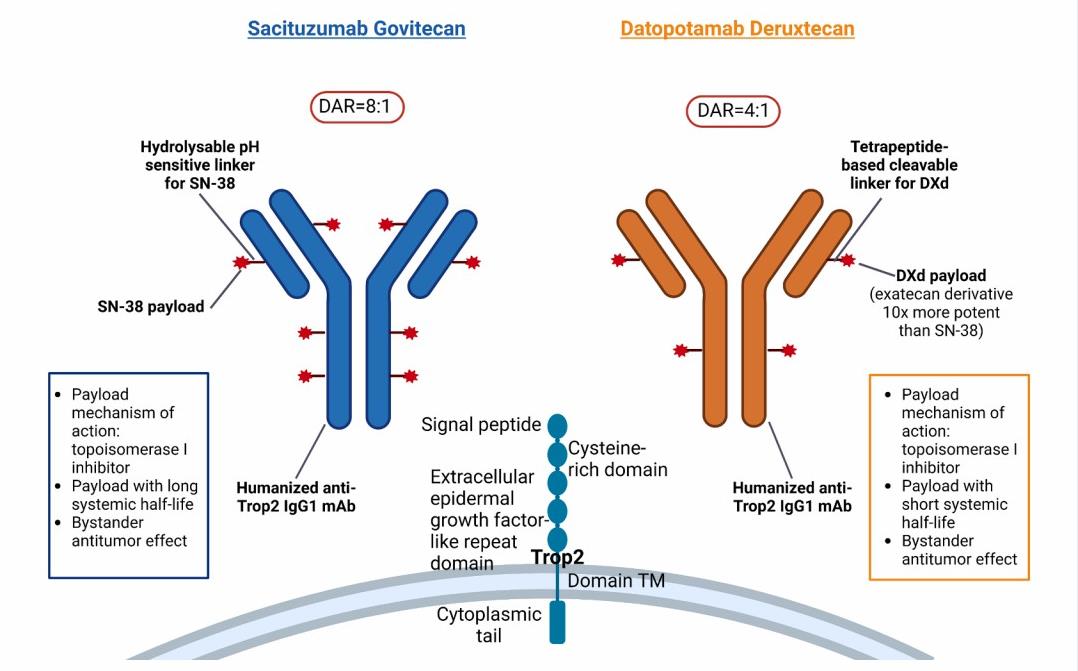 Fig. 2. ADCs targeting TROP-2: Sacituzumab Govitecan and Datopotamab Deruxtecan.(Parisi C, et al., 2018)
Fig. 2. ADCs targeting TROP-2: Sacituzumab Govitecan and Datopotamab Deruxtecan.(Parisi C, et al., 2018)
Sacituzumab Govitecan
- Sacituzumab govitecan (IMMU-132, SG), a first-in-class TROP-2-directed ADC, is linked to the cytotoxic payload SN-38 (7-ethyl-10-hydroxycamptothecin), the active metabolite of topoisomerase I inhibitor irinotecan, via a hydrolyzable linker.
- SG is characterized by a high drug-to-antibody ratio (DAR) and the release of SN-38 from the antibody via a bystander effect without the need for internalization and enzymatic cleavage by tumor cells.
- A first-in-human phase I trial of SG showed good antitumor activity in heavily pretreated patients with diverse metastatic solid tumors not pre-selected for Trop-2 expression.
- In another study, SG demonstrated a manageable safety profile, with the most common of all grades' AEs being nausea (80%), diarrhea (61%), fatigue (46%), alopecia (39%) and neutropenia (37%). Neutropenia was the most common among grade 3–4 AEs (28%), but the rate of febrile neutropenia was low (4%).
- SG has been approved by the FDA for metastatic TNBC, hormone receptor positive, HER2-ve metastatic breast cancer, and it was granted accelerated approval for metastatic urothelial cancer.
Datopotamab Deruxtecan
- Datopotamab Deruxtecan (Dato-DXd, DS-1062) is a humanized TROP-2-directed antibody linked to a topoisomerase 1 inhibitor payload (exatacan derivative) via a tetrapeptide-based cleavable linker. The linker releases DXd after proteolytic processing by lysosomal proteases such as cathepsins.
- In preclinical studies, Dato-DXd remarkably reduced the growth of TROP-2-high cell lines but was not effective in inhibiting cell growth with low TROP-2 levels and induced tumor inhibition in TROP-2-high NSCLC PDX models.
- In a phase 1 study, Dato-DXd was administered across different doses (4.0 mg/kg n = 50, 6.0 mg/kg n = 50, 8.0 mg/kg n = 80) every 3 weeks (Q3W) in pretreated NSCLC patients (n = 34). An obvious antitumor activity and responses that were generally durable were observed. Drug-related grade ≥ 3 AEs occurred in 14%, 26%, and 35%, respectively at the dose of 4, 6, and 8 mg/Kg.
- In a pharmacometric analysis, the incidence of dose reduction and grade ≥ 2 stomatitis/mucositis were positively correlated with exposure.
For NSCLC, the phase I/II IMMU-132-01 basket trial with the TROP2-targeted ADC SG demonstrated activity in NSCLC. The phase I TROPION-PanTumor01 trial with datopotamab deruxtecan displayed promising response rates for heavily pretreated advanced NSCLC, including patients with treated CNS metastases. Therefore, there are promising results in patients with advanced or metastatic NSCLC using TROP2-targeted ADCs, and testing for TROP-2 in advance of treatment with TROP2-targeted ADCs is not warranted.
Reference
- Parisi C, Mahjoubi L, Gazzah A, et al. TROP-2 directed antibody-drug conjugates (ADCs): The revolution of smart drug delivery in advanced non-small cell lung cancer (NSCLC). Cancer Treat Rev. 2023, 118:102572.
For Research Use Only. NOT FOR CLINICAL USE.

Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
Contact usUSA
Tel:
Fax:
Email:
Europe
Tel:
Email:
Germany
Tel:
Email:

