Development and Evaluation of MMAE-Trastuzumab ADCs
Trastuzumab & Breast Cancer
Although trastuzumab has been approved as standard therapy for the treatment of patients with HER2-overexpression breast cancer, patients treated with trastuzumab can experience some serious adverse effects. Therefore, new approaches are needed to improve the therapeutic index of trastuzumab to make treatment more effective, and MMAE-trastuzumab ADCs are a potential pathway.
Creative Biolabs has extensive experience in the field of developing ADCs and provides ADC development for breast cancer.
MMAE-Trastuzumab ADC Preparation
In the study, the antibody, the linker, and the drug are respectively trastuzumab, MC-VC-PAB, and MMAE. What is shows in Fig. 1 is the structure of the resultant MMAE-trastuzumab ADCs.
First, the researchers converted the trastuzumab buffer to borate buffer and treated it with DTT. Next, excess DTT molecules were removed by ultrafiltration, the concentration of reduced trastuzumab was adjusted by degassed PBS-D, and then reduced trastuzumab was cooled on ice. Shortly afterwards, the concentration of free thiols was measured by Ellman's reagent. Subsequently, DMSO was slowly added to the solution, and the mixture was incubated for 1 min under continuous agitation. Each trastuzumab was alkylated drop-by-drop with drug/linker on ice with continuous stirring. Cysteine was added to quench the unreacted drug/linker molecules, and then the MMAE-trastuzumab ADC was purified and concentrated. Finally, MMAE-Trastuzumab ADCs were stored at -80℃.
 Fig. 1 Development of MMAE-trastuzumab ADCs. (Yaghoubi, S., et al.,2021)
Fig. 1 Development of MMAE-trastuzumab ADCs. (Yaghoubi, S., et al.,2021)
Creative Biolabs provides one-stop ADC development services with complete and scientific solutions according to your requirements.
MMAE-Trastuzumab ADC Characterization
The study indicated the characterization of MMAE-trastuzumab ADCs in five aspects, including reduction and alkylation confirmation, SDS-PAGE analysis, determination of the binding potential of MMAE-trastuzumab ADCs by ELISA, cytotoxicity assay, and assessment of the effect of MMAE-trastuzumab ADCs on cell proliferation.
SDS-PAGE Analysis
As shown in Fig. 2A (chains 2 and 3), unconjugated trastuzumab and MMAE-trastuzumab ADCs produced only two bands under reducing conditions, representing the heavy (H) and light (L) chains of 50 and 25 kDa, respectively. In nonreducing SDS-PAGE, unconjugated trastuzumab showed only one band corresponding to H2L2 at approximately 150 kDa, representing intact interchain disulfide bonds (FIG. 2A, lane 3). MMAE - trastuzumab ADCs have 6 bands, respectively, at 150, 125, 100, 75, 50, and 25 kDa, representing H2L2, H2L, H2, the HL, H, and L, reflecting the characteristics of the drug/linked distribution.
Determination of the Binding Potential of MMAE-Trastuzumab ADCs by ELISA
Unconjugated trastuzumab and MMAE-trastuzumab ADCs had similar binding abilities to cell-surface antigens (Fig. 2B). This indicated that the binding capacity of ADC was not significantly affected by conjugation compared with unconjugated trastuzumab.
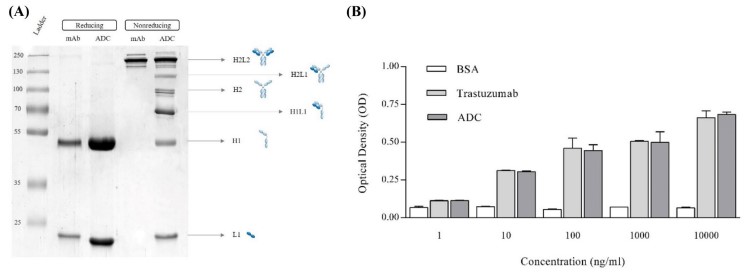 Fig. 2 ADC characterization. (A) SDS-PAGE. (B) Effects of MMAE conjugation on trastuzumab binding capacity through cell-based ELISA. (Yaghoubi, S., et al.,2021)
Fig. 2 ADC characterization. (A) SDS-PAGE. (B) Effects of MMAE conjugation on trastuzumab binding capacity through cell-based ELISA. (Yaghoubi, S., et al.,2021)
Cytotoxicity Assay
MMAE-trastuzumab ADCs caused significant cell death in MDA-MB-453 cells (her2-positive cells) after 48h and 72h, resulting in a dose-dependent growth arrest of the G2/M cell cycle. However, monoclonal trastuzumab had a small amount of inhibitory activity against MDA-MB-453 cells. In contrast, neither trastuzumab nor MMAE-trastuzumab ADCs had an inhibitory effect or a minor inhibitory effect on HEK293 (her2-negative) cells.
Assessment of the Effect of MMAE-Trastuzumab ADCs on Cell Proliferation
Compared with the untreated group, the colony-forming ability of MDA-MB-468 cells in the MMAE-Trastuzumab ADCs group was considerably decreased. However, a slight decrease in colony formation was detected when the MMAE-Trastuzumab ADCs were treated with HEK293 cells (Fig. 3).
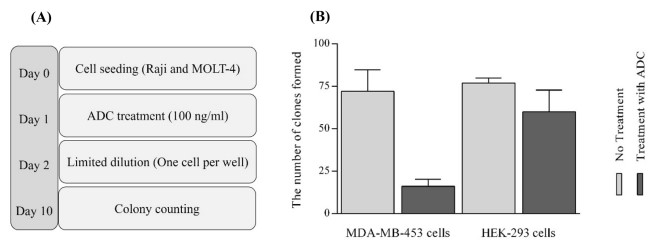 Fig. 3 ADC characterization. (A) Schematic diagram of the steps used for colony formation assay. (B) Assessment of MMAE-trastuzumab ADCs on cell proliferation. (Yaghoubi, S., et al.,2021)
Fig. 3 ADC characterization. (A) Schematic diagram of the steps used for colony formation assay. (B) Assessment of MMAE-trastuzumab ADCs on cell proliferation. (Yaghoubi, S., et al.,2021)
Based on our mature antibody engineering platform, we can provide anti-HER2 antibody development services catering to specific customer requirements, along with an ample supply of pre-made anti-HER2 ADC products to advance ADC drug development. Collaborate with us to learn more about the strategies for anti-HER2 ADC development.
Reference
- Yaghoubi, S., et al., Development and biological assessment of mmae-trastuzumab antibody-drug conjugates (adcs). Breast Cancer, 2021. 28(1): p. 216-225.
For Research Use Only. NOT FOR CLINICAL USE.

Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
Contact usUSA
Tel:
Fax:
Email:
Europe
Tel:
Email:
Germany
Tel:
Email:

