- Home
- UTC Development
- Antibody-Antibiotic Conjugate (AAC) Development
- Antibiotic Synthesis
- dmDNA31 Synthesis
dmDNA31 Synthesis Service
As an emerging therapeutic approach for drug-resistant bacterial Infection, antibody-antibiotic conjugates (AACs) combine the advantages of antibodies and antibiotics to achieve the target delivery of the antibiotic payload to the infection site. With a deep understanding of the antibiotic structure, chemistry, and effects, Creative Biolabs provides customized AACs development services using dmDNA31 as payloads.
Introduction
Rifampicin, also known as rifampin, was discovered in 1965 and approved in the United States in 1971. It is the most effective and safe medicines needed in a health system on the World Health Organization's List of Essential Medicines. Rifampicin is a potent antibiotic used to treat several types of bacterial infections, including tuberculosis, leprosy, mycobacterium avium complex, Legionnaire's disease and so on. It is almost always used along with other antibiotics. The dmDNA31 (dimethyl DNA31, 4-dimethylamino piperidino-hydroxybenzoxazinorifamycin) is a rifalazil analog, referred to as rifalog. It has effective bactericidal activity against persisters and stationary-phase S. aureus and is well retained in macrophages. Thus, the antibiotic must be able to kill dormant bacteria and harbor prolonged cellular retention for S. aureus infections.
Mode of Action of dmDNA31
Rifampicin harbors the ability to inhibit bacterial DNA-dependent RNA synthesis by inhibiting bacterial DNA-dependent RNA polymerase. dmDNA31/Rifampicin binds to the pocket of the RNA polymerase β subunit within the DNA/RNA channel according to previous crystal structure data and biochemical data suggested, but away from the active site. The inhibitor restrains RNA synthesis by physically blocking elongation of RNA, and therefore preventing synthesis of host bacterial proteins. By this "steric-occlusion" mechanism, dmDNA31/Rifampicin blocks synthesis of the second or third phosphodiester bond between the nucleotides in the RNA backbone, preventing elongation of the 5' end of the RNA transcript past more than 2 or 3 nucleotides.
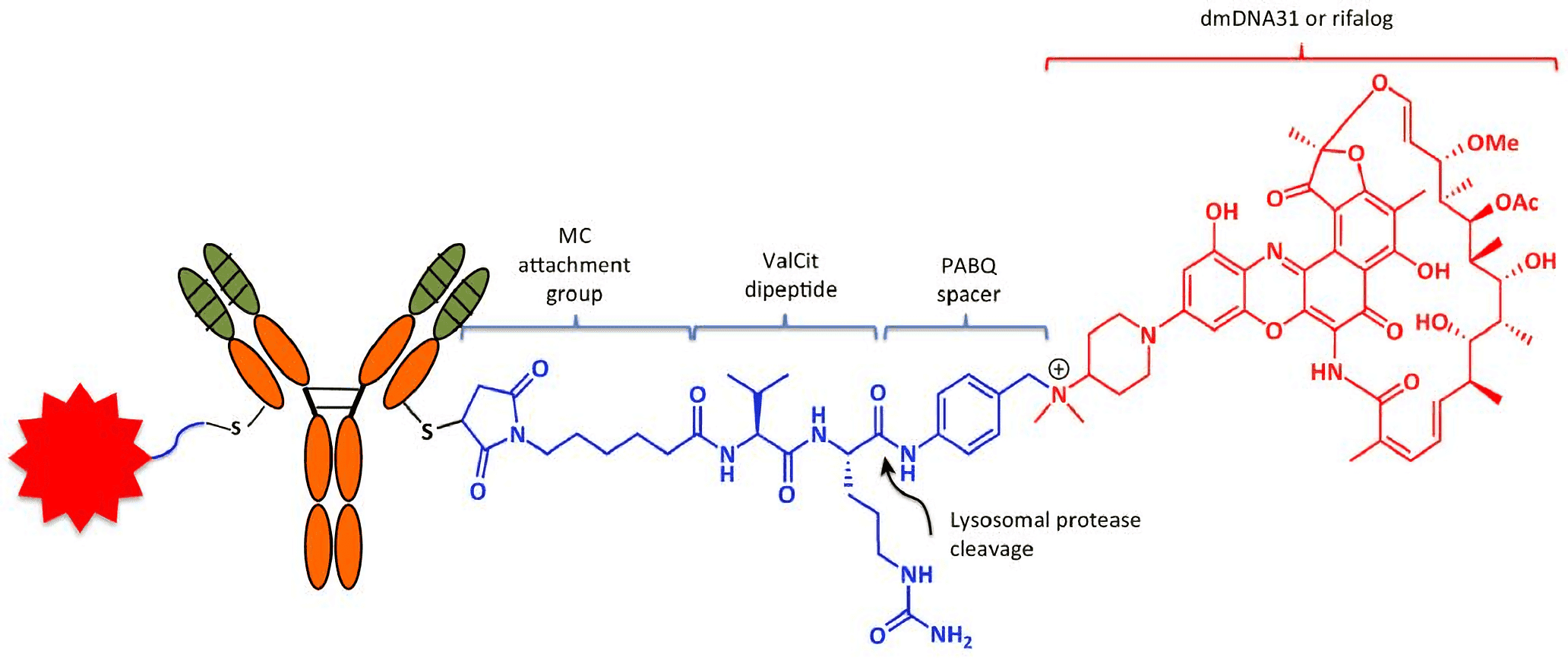 Fig.1 Anatomy of the Antibody–antibiotic conjugate (AAC). Each anti-S. aureus AAC consists of two molecules: dmDNA31 antibiotics (red) linked to each of the light chains of the β-GlcNAc-WTA monoclonal antibody (mAb) via an MC-ValCit-PABQ linker (blue; the red star on the left of the AAC is another linker-drug moiety). Attachment to the mAb is achieved through covalent linkage between the reactive thiol in the engineered cysteine of the antibody and maleimide. The linker–antibiotic attachment is formed between the self-immolation p-aminobenzyl quaternary ammonium salt (PABQ) and the tertiary amine of dmDNA31. Within acidic intracellular vesicles, lysosomal proteases such as cathepsin B cleave the amide bond (arrowhead) at the C terminus of citrulline to release active dmDNA31 following the self-immolation of PABQ. (Mariathasan, 2017)
Fig.1 Anatomy of the Antibody–antibiotic conjugate (AAC). Each anti-S. aureus AAC consists of two molecules: dmDNA31 antibiotics (red) linked to each of the light chains of the β-GlcNAc-WTA monoclonal antibody (mAb) via an MC-ValCit-PABQ linker (blue; the red star on the left of the AAC is another linker-drug moiety). Attachment to the mAb is achieved through covalent linkage between the reactive thiol in the engineered cysteine of the antibody and maleimide. The linker–antibiotic attachment is formed between the self-immolation p-aminobenzyl quaternary ammonium salt (PABQ) and the tertiary amine of dmDNA31. Within acidic intracellular vesicles, lysosomal proteases such as cathepsin B cleave the amide bond (arrowhead) at the C terminus of citrulline to release active dmDNA31 following the self-immolation of PABQ. (Mariathasan, 2017)
dmDNA31-based AACs
Base on the specificity of antimicrobial monoclonal antibody, AACs using dmDNA31 as payloads can achieve target delivery of dmDNA31 to the infection site, thereby reducing collateral damage to the normal tissues. β-GlcNAc-WTA mAb is a humanized monoclonal antibody and its target is β-GlcNAc residues which are on the ribitol phosphate units of wall teichoic acid of bacteria. By combining the cytotoxic dmDNA31 with β-GlcNAc-WTA mAb, Creative Biolabs can design an appropriate linker to generate β-GlcNAc-WTA mAb-dmDNA31 AAC for the therapy of Methicillin-resistant Staphylococcus aureus (MRSA).
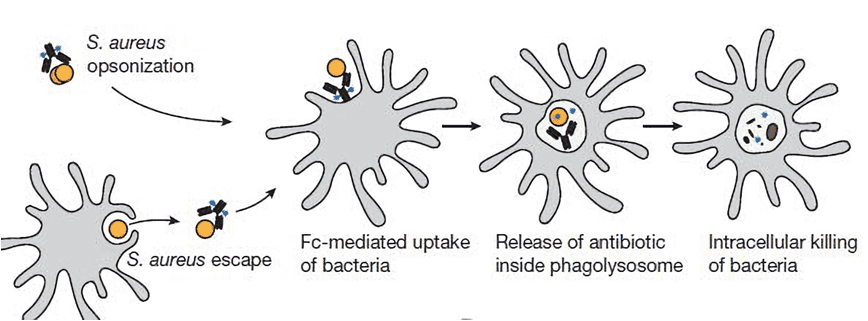 Fig.2 Mechanism of AAC action. AAC has no direct antibacterial activity when bound to planktonic S. aureus and does not diffuse into mammalian cells, owing to the size of the antibody. However, when AAC-opsonized bacteria are taken up by host cells, intracellular proteases cleave the linker and readily release the antibiotic in its active form. (Lehar, 2015)
Fig.2 Mechanism of AAC action. AAC has no direct antibacterial activity when bound to planktonic S. aureus and does not diffuse into mammalian cells, owing to the size of the antibody. However, when AAC-opsonized bacteria are taken up by host cells, intracellular proteases cleave the linker and readily release the antibiotic in its active form. (Lehar, 2015)
With our well-established synthesis platform and experienced scientists at Creative Biolabs, we are dedicated to helping you develop dmDNA31-linker complexes using customized linkers for antibody conjugation in a timely and cost-effective method. We believe our high-quality services and products will contribute greatly to the success of your projects. Please feel free to contact us for more information and a detailed quote.
References:
- Mariathasan, S, Tan, M. W. (2017). “Antibody-antibiotic conjugates: a novel therapeutic platform against bacterial infections”. Trends in Molecular Medicine, 23(2), 135.
- Lehar, S. M.; et al. (2015). “Novel antibody–antibiotic conjugate eliminates intracellular s. aureus”. Nature, 527(7578), 323-328.
For Research Use Only. NOT FOR CLINICAL USE.

Online Inquiry
Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
