- Home
- UTC Development
- Fragment-Drug Conjugate Development
- Antibody Fragment-Drug Conjugate Development
- ScFv-Drug Conjugate Development
ScFv-Drug Conjugate Development Service
Advances in antibody engineering technology are enabling the designation of single chain fragment variable (scFv) for specific purposes in fragment-drug conjugate (FDC) development. With years of antibody expression and conjugation experience, Creative Biolabs has established a series of approaches for scFv-drug conjugate construction. We now provide personalized scFv-baesd FDC development services for global clients to meet your specific project demands.
Introduction of ScFv
Fragment variable (Fv) is the smallest unit of immunoglobulin (Ig) molecule with function in antigen-binding activities. An antibody in scFv format consists of variable regions of heavy (VH) and light (VL) chains, which are joined together by a flexible peptide linker that can be easily expressed in bacterial, plant, yeast, and mammalian cell systems, allowing protein engineering to improve the properties of scFv such as the increase of affinity and alteration of specificity.
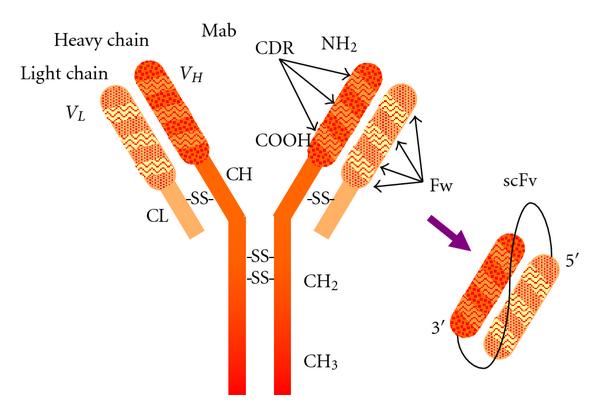 Fig.1 Antibody model showing subunit composition and domain distribution.1,2
Fig.1 Antibody model showing subunit composition and domain distribution.1,2
Diabodies are comprised of two scFv fragments associated to form a ∼60 kDa bivalent dimer complex. It is possible to form bispecific heterodimers and bivalent homodimers. Diabody expression is similar to that of scFvs, though crucially shorter linkers between the heavy and light chain Fv fragments are used (normally less than 12 residues). These shorter linker lengths inhibit efficient folding of the Fv fragment into an scFv, and instead force the formation of a non-covalent diabody complex. Tandem scFv is produced by connecting two scFv molecules through a short linker.
In the scFv construction, the order of the domains can be either VH-linker-VL or VL-linker-VH and both orientations have been applied. However, most of the scFv are constructed in a VH-linker-VL orientation. The most popular methods used for scFV generation are polymerase chain reaction (PCR) assembly and combinatorial transfection. ScFv expression facilitates rapid production in large quantities. Besides, the small size of scFvs makes them ideal candidates for high-throughput selection technologies such as phage display and yeast display. Through multiple rounds of selection, high-binding clones against target can be identified and expressed.
Conjugation Strategy
Cysteine Conjugation
Multiple payloads have been explored in the generation of scFv-based FDC, including doxorubicin, monomethyl auristatin E (MMAE), and monomethyl auristatin F (MMAF). Since scFv fragments are most commonly produced via expression, the process of site-selective payload conjugation is simplified. Reactive amino acids can easily be introduced to specific locations on the scFv fragment using site-directed mutagenesis and subsequently exploited for highly-controlled payload conjugation. The most popular choice of amino acid in this regard is cysteine due to the wide range of chemical linkers designed with cysteine selectivity such as maleimide. For instance, a diabody against CD30 was engineered with four site-specific cysteine residues, which were subsequently conjugated to MMAE and MMAF payloads via cysteine-selective maleimide chemistry.
Development of scFv-based FDCs with great potential involves the optimization of the following four critical parameters.
- Selecting suitable target antigen
- Using high-binding clones
- Using highly potent drugs that are attenuated and stable while attached to the scFv fragment
- Using linkers that allow for the release of active drug only when the targeting scFv has reached the target site
With rich experience in protein expression and ADC development, Creative Biolabs now provides customized scFv-based FDC development services for our clients with various conjugation approaches. If you are interested in our services, please feel free to contact us for more information.
Related Services
- Fab-drug Conjugates Development
- SIP-drug Conjugates Development
- sdAb-drug Conjugates Development
- Scaffold-drug Conjugates Development
References
- Ahmad, Zuhaida Asra, et al. "scFv antibody: principles and clinical application." Journal of Immunology Research 2012.1 (2012): 980250.
- Distributed under Open Access License CC BY 4.0, without modification.
For Research Use Only. NOT FOR CLINICAL USE.

Online Inquiry
Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
