ADC Development Services Targeting RON
Aberrant expression of the RON receptor tyrosine kinase in breast cancer (BC) and non-small cell lung cancer (NSCLC) has therapeutic implication. Anti-RON monoclonal antibody (mAb) directed delivery of the highly potent drug in the form of antibody-drug conjugate (ADC) is a therapeutic strategy for targeted treatment of NSCLC cancer. Scientists at Creative Biolabs have extensive experience in providing services related to the synthesis and characterization of ADC. We have worked with a variety of highly potent payloads, as well as a selection of cleavable and non-cleavable linkers and spacers. At present, we provide customized service for your anti-RON ADC development project.
Introduction of RON
RON is a member of the MET proto-oncogene family implicated in the pathogenesis of various cancers including those from breast, colon, lung, and pancreas. The oncogenic potential of RON is represented by its capacity to induce migration, invasion, growth, survival and epithelial-mesenchamal transition of epithelial tumor cells, as well as to promote pro-tumoral activities of tumor-associated macrophages. Originally synthesized as a single chain precursor, RON is processed into a disulfide-linked heterodimer with a transmembrane β-chain and an extracellular α-chain. The intracellular region of RON includes three parts, the juxtamembrane domain, the highly conserved kinase domain and the non-catalytic C-terminal tail. The kinase domain consists of two lobes, the N-lobe and the C-lobe.
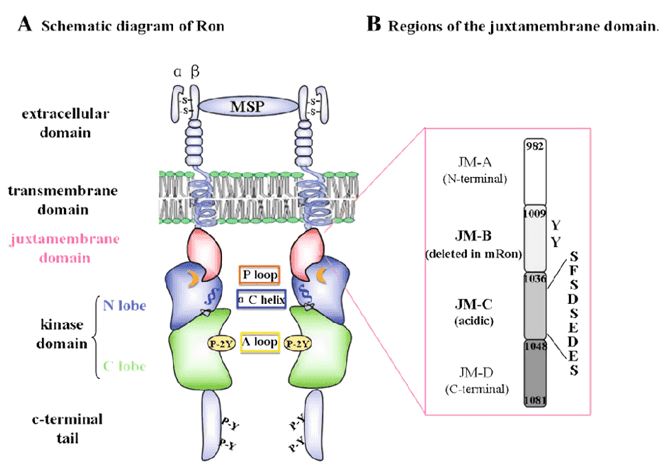 Fig.1 Schematic diagram of the RON receptor.1,2
Fig.1 Schematic diagram of the RON receptor.1,2
Overexpression of RON has been considered as a potential drug target for various cancer treatment. Currently, RON-specific therapeutics including tyrosine kinase inhibitors (TKI) and therapeutic mAbs have been developed and validated in various preclinical cancer models. However, due to the lack of complete addiction of cancer cells to RON signaling for growth and survival, the efficacy of RON- specific TKIs and therapeutic mAbs is relatively low with only partial tumor inhibition. Thus, it is critical to improve the drug efficacy for the success of RON-targeted cancer therapy.
What Can We Do for You?
Our basic selection criteria of antibodies for drug conjugation and delivery is based on its unique feature in induction of RON internalization. Equipped with perfect antibody preparation platform and unique ADC Antibody Screening platform, a series of mAbs specific to human RON can be developed for RON-targeted drug delivery. Creative Biolabs has over a decade of working experience in mAb modification to fit various bio-conjugation strategies. Technicists at Creative Biolabs are experienced in performing Lysine Conjugation, Cysteine Conjugation as well as other site-specific Conjugation for ADC development. If you are interested in our ADC development services against RON, please don't hesitate to contact us for more information.
References
- Wang, Xin, Neela Yennawar, and Pamela A. Hankey. "Autoinhibition of the Ron receptor tyrosine kinase by the juxtamembrane domain." Cell Communication and Signaling 12 (2014): 1-10.
- Distributed under Open Access License CC BY 4.0, without modification.
For Research Use Only. NOT FOR CLINICAL USE.

Online Inquiry
Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
