- Home
- UTC Development
- Antibody-Enzyme Conjugate Development
- Antibody-directed Toxic Enzyme Fusion Protein Development
- Antibody-Barnase Fusion Protein
Antibody-Barnase Fusion Protein Development Service
As a professional service provider in antibody-drug conjugates (ADCs) design and preparation, Creative Biolabs provides comprehensive customized antibody-barnase fusion protein for targeted immunotherapy with optimized enzyme and antibody conjugation strategies.
Barnase
Barnase is a bacterial RNase synthesized and secreted by Bacillus amyloliquefaciens, which consists of 110 amino acids and has ribonuclease activity. It shares 84% sequence identity with binase (derived from Bacillus intermedius), both of which have structures representative of the T1 RNase family that preferentially inactivates the large ribosomal subunit. Barnase is lethal to the cell when expressed without its inhibitor barstar. Barnase has been found to be highly toxic to cancer cells as a result of its resistance to the RNase inhibitor (RI) and, in addition, it has a high catalytic activity and stability, in contrast to human RNases. Barnase catalyzes hydrolysis at diribonucleotide GpN sites in two steps. The most important catalytic residues are Glu73 and His102 in catalysis.
Antibody-barnase Fusion Protein
Microbial RNases are of potential functions as novel anticancer agents, although they present the possibility of inducing immunoreactions in humans. The fusion of antibody and RNases confers the advantage of tumor specificity to the fusion protein is under development actively. Fusion of anti‑HER2 (4D5) antibody to two barnase molecules resulted in a threefold increase in cytotoxicity of the fusion protein to induce apoptosis in human ovarian carcinoma cells compared with free barnase. In addition, in vivo studies demonstrated that 4D5-dibarnase could cause a significant reduction in the growth of breast carcinoma xenografts (about 76%), and an increase in the dosage of the immunoRNase did not appear to cause nonspecific toxicity to the mice.
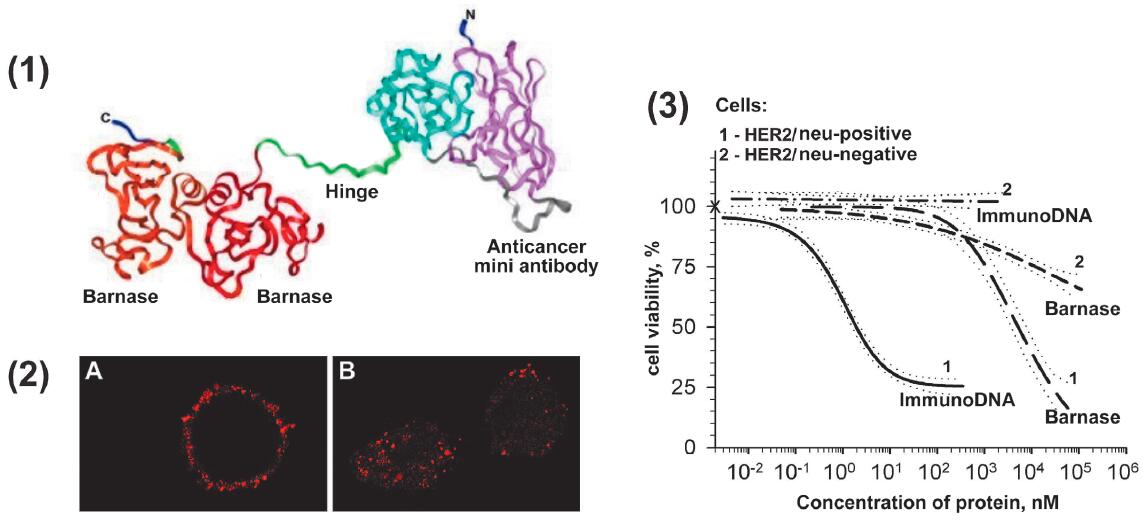 Fig.1 Immunodibarnase as a perspective agent for treating malignant neoplasms.1,2
Fig.1 Immunodibarnase as a perspective agent for treating malignant neoplasms.1,2
Creative Biolabs has the ability to provide customers the best services concerning the antibody-barnase fusion protein. Our experienced scientists are dedicated to helping you develop specific antibody-fusion protein in a timely and cost-effective manner. Please feel free to contact us for more information and a detailed quote.
References
- Deyev, S. M., and E. N. Lebedenko. "Modern technologies for creating synthetic antibodies for clinical application." Acta Naturae (англоязычная версия) 1.1 (1) (2009): 32-50.
- Distributed under Open Access License CC BY 4.0, without modification.
For Research Use Only. NOT FOR CLINICAL USE.

Online Inquiry
Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
