Development and Evaluation of an Anti-CD22-Seco-CBI-Dimer ADC for the Treatment of NHL
New Generation ADC & Non-Hodgkin Lymphoma
Clinical trial data suggest that ADCs containing the microtubule inhibitor MMAE, such as pinatuzumab vedotin (anti-CD22-vc-MMAE) and polatuzumab vedotin (anti-CD79b-vc-MMAE), show promising efficacy for the treatment of non-Hodgkin lymphoma(NHL). However, some patients do not respond or become resistant to these ADCs. Studies have shown that resistance to ADCs is driven by resistance to the MMAE payload rather than target antigen loss. It is envisioned that an ADC with a different linker drug might provide a longer duration of response and work in tumors resistant to MMAE ADCs. Therefore, the development and evaluation of a new generation of antibody conjugates, specifically an ADC with seco-CBI-dimer payloads, for NHL treatment holds significant for clinical promise.
Creative Biolabs has extensive experience in the field of ADC development and provides a comprehensive range of services for ADC development for NHL.
In this study, an anti-CD22 ADC with a seco-CBI-dimer payload, thio-Hu anti-CD22-(LC: K149C)-SN36248 was tested and compared with pinatuzumab vedotin for its efficacy and duration of response in xenograft models, as well as its ability to deplete normal B cells in cynomolgus monkeys. SN36248, a noncleavable linker drug combination, has previously demonstrated high in vivo activity in xenograft models of B-cell lymphomas, while the K149C conjugation site in the light chain offers additional in vivo stability to the payload.
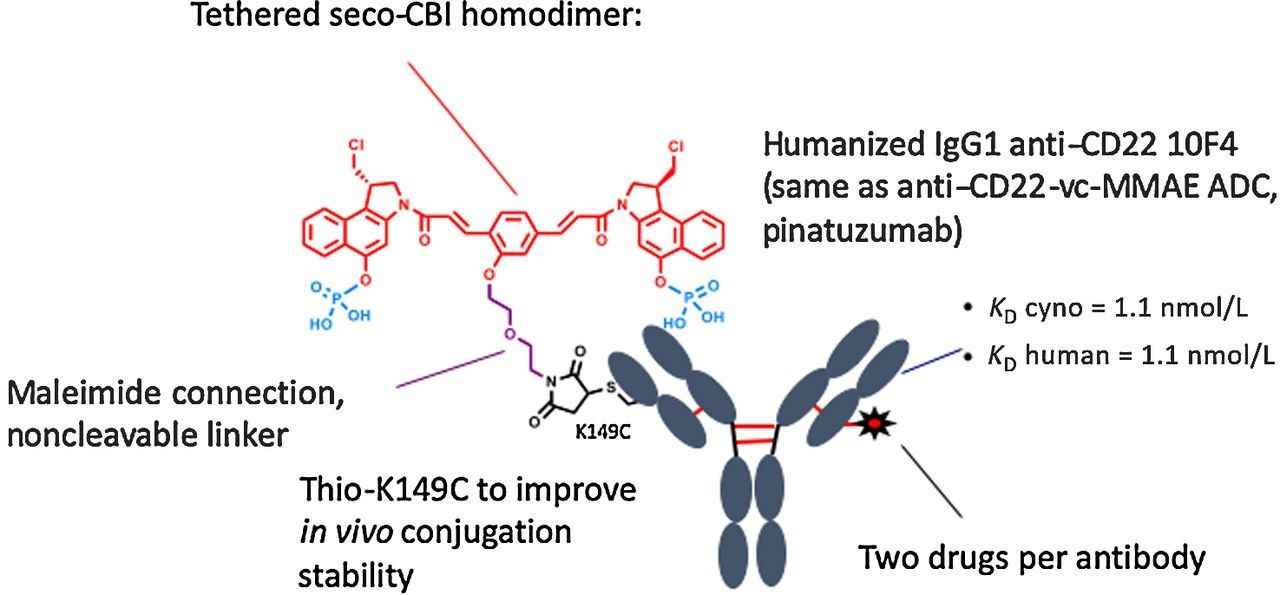 Fig. 1 Structure of anti-CD22-SN36248.1
Fig. 1 Structure of anti-CD22-SN36248.1
Creative Biolabs provides one-stop ADC development services according to your needs, and excels in utilizing various non-cleavable linkers to develop exceptional ADCs.
Efficacy of Thio-Hu Anti-CD22-(LC:K149C)-SN36248
Subcutaneous xenograft models were established using multiple human NHL cell lines (BJAB.Luc, BJAB.Luc-22R1.2, WSU-DLCL2, and Raji) to evaluate the activity of anti-CD22-SN36248 and pinatuzumab vedotin. Anti-CD22-SN36248 demonstrated high activity in all three models in a target- and dose-dependent manner (Fig. 2A-C).
- In the BJAB.Luc model, pinatuzumab vedotin achieved tumor regression at a dose of 1.5 mg/kg. Anti-CD22-SN36248 exhibited strong antitumor activity in this model, with a single dose of 0.3 mg/kg effectively eliminating the tumors.
- In the Raji and WSU-DLCL2 models where pinatuzumab vedotin was less effective, while anti-CD22-SN36248 displayed robust efficacy, and a single dose of 1 mg/kg was sufficient to achieve regression in these models.
- In the BJAB.Luc-22R1.2 xenograft model, which exhibited resistance to both pinatuzumab vedotin and polatuzumab vedotin, anti-CD22-SN36248 remained highly effective at 1 mg/kg, comparable to the efficacy of pinatuzumab vedotin in the parental cell line.
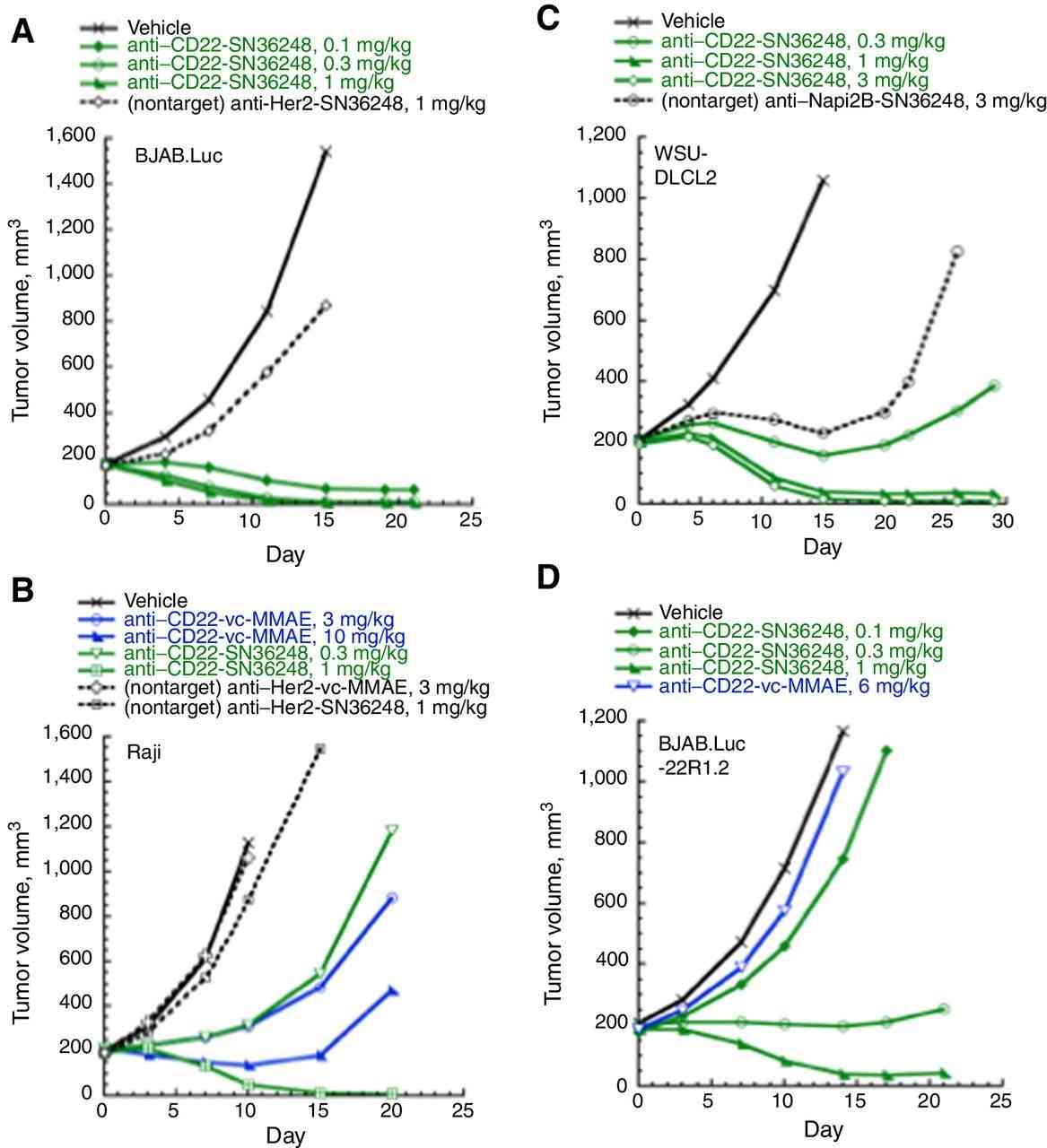 Fig. 2 In vivo efficacy of anti-CD22-SN36248 in tumor xenograft models of (A) BJAB.Luc, (B) Raji, (C) WSU-DLCL2, and (D) BJAB.luc-22R1.2 human NHL.1
Fig. 2 In vivo efficacy of anti-CD22-SN36248 in tumor xenograft models of (A) BJAB.Luc, (B) Raji, (C) WSU-DLCL2, and (D) BJAB.luc-22R1.2 human NHL.1
The Duration of Response Study of Anti-CD22-SN36248 and Pinatuzumab Vedotin
The WSU-DLCL2 xenograft model was selected to evaluate the duration of response. The majority of tumors treated with pinatuzumab vedotin exceeded twice their initial size by day 90, while most tumors treated with anti-CD22-SN36248 showed no apparent progression (i.e., no increase from their starting size) by the end of the study on day 130. These data suggest that repeat dosing of anti-CD22-SN36248 and pinatuzumab vedotin was well-tolerated in animals and anti-CD22-SN36248 yielded a more durable response than pinatuzumab vedotin (Fig. 3).
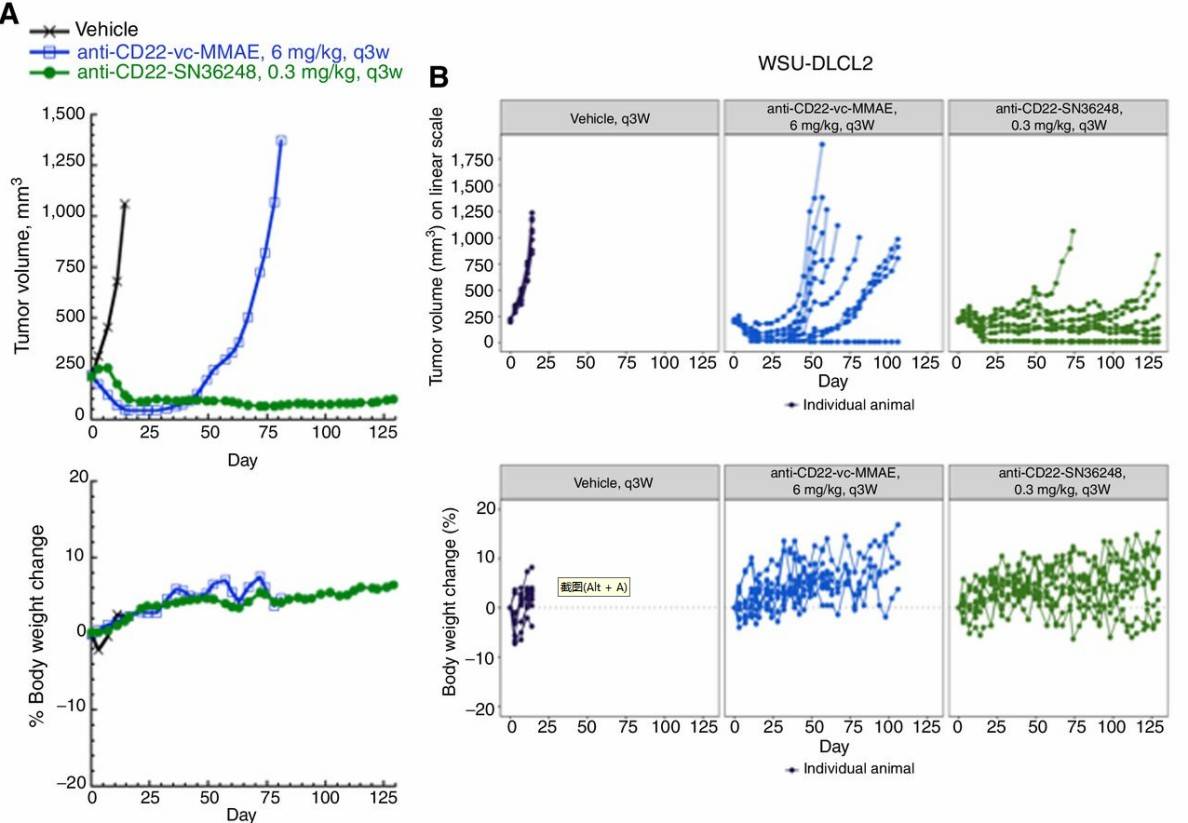 Fig. 3 Durable response to anti-CD22-SN36248 in the WSU-DLCL2 tumor xenograft model.1
Fig. 3 Durable response to anti-CD22-SN36248 in the WSU-DLCL2 tumor xenograft model.1
Mechanism of Resistance to Anti-CD22-SN3624
For the assessment of resistance mechanisms to anti-CD22-SN36248, a sensitive model, BJAB.Luc, and derived sublines resistant to anti-CD22-SN36248 (Fig. 4A and B) were selected. Researchers initially treated the parental xenograft tumor with a suboptimal dose (0.15 mg/kg) of anti-CD22-SN36248. Upon tumor regrowth of, mice received a higher (though still suboptimal) dose. Tumors from mice that received doses between 4 and 6 mg/kg (shown in Fig. 4A) were harvested, fractionated, and reimplanted into naive mice for ongoing selection. Tumors were selected based on their resistance to two doses of 6 mg/kg anti-CD22-SN36248 (shown in Fig. 4B) and then cultured to derive cell lines for further analysis using FACS. Most of the drug-resistant cell lines, except R488, still expressed CD22. Subsequently, researchers conducted RNA sequencing (RNA-seq) analysis on these resistant cell lines and found upregulation of P-gp in only one line (R491). P-gp expression was confirmed by FACS analysis (Fig. 4E), but the analysis of RNA-seq data did not reveal any other apparent mechanisms of resistance. These results indicate that resistance mediated by P-gp is specific to the payload, and the active catabolite released in the cells from anti-CD22-SN36248 is likely the same as that from anti-CD22-SN36325, the CBI-dimer payload.
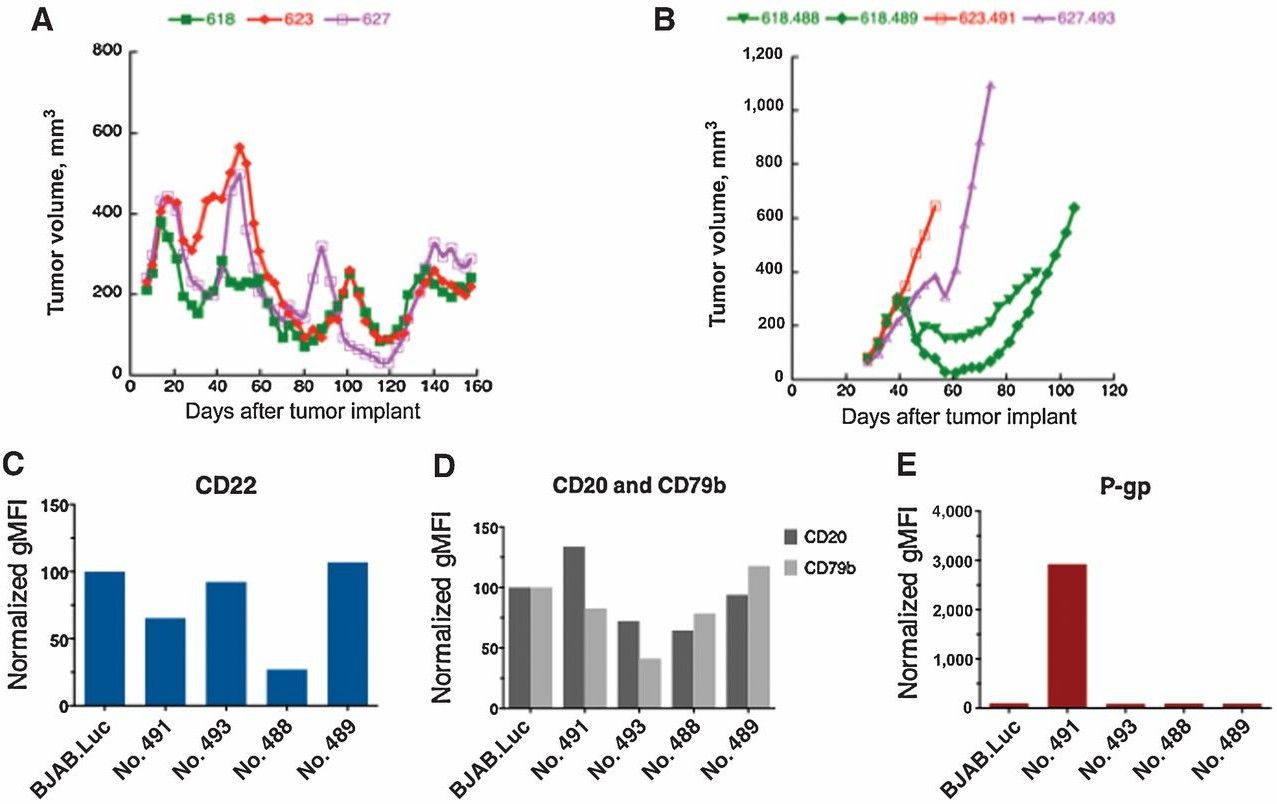 Fig. 4 Mechanism of resistance to anti-CD22-SN36248.1
Fig. 4 Mechanism of resistance to anti-CD22-SN36248.1
PK/PD Study of Anti-CD22-SN36248 in Cynomolgus Monkey
Cynomolgus monkeys aged between 2.6 and 4 years, weighing between 2.4 and 4 kg, were selected for dosing to investigate the exposure-response relationship related to B-cell depletion. Throughout the study, blood was collected at various time points for pharmacokinetic (PK), pharmacodynamic (PD), immunophenotyping via flow cytometry, B-cell-activating factor (BAFF) analysis, and clinical pathology (hematology and clinical chemistry). At study's conclusion, animals were euthanized for complete necropsy examination, and tissues were processed for histology. An enzyme-linked immunosorbent assay (ELISA) was employed to measure BAFF levels in serum from cynomolgus monkeys.
There were no observed ADC-related clinical signs or effects on food consumption, body weights, serum chemistry parameters, or gross pathology. Anti-CD22-SN36248 exhibited dose-proportional total antibody Cmax values and greater than dose-proportional total antibody AUC0-inf across the dose range tested. In peripheral blood, antigen-dependent reductions in B lymphocytes were observed. Researchers also monitored serum BAFF levels, another measure of B-cell depletion, which inversely correlated with B-cell numbers. Dose-dependent reductions of B lymphocytes in the lymph node as measured by FACS, were observed (Fig. 5C). Immunohistochemistry (IHC) staining of CD20, a B-cell marker, in the mesenteric lymph node and spleen confirmed a persistent on-target effect of targeting CD22 (Fig. 5D). This result contrasts with the performance of pinatuzumab vedotin, suggesting that anti-CD22-SN36248 could be more effective than pina or pola in more indolent NHLs.
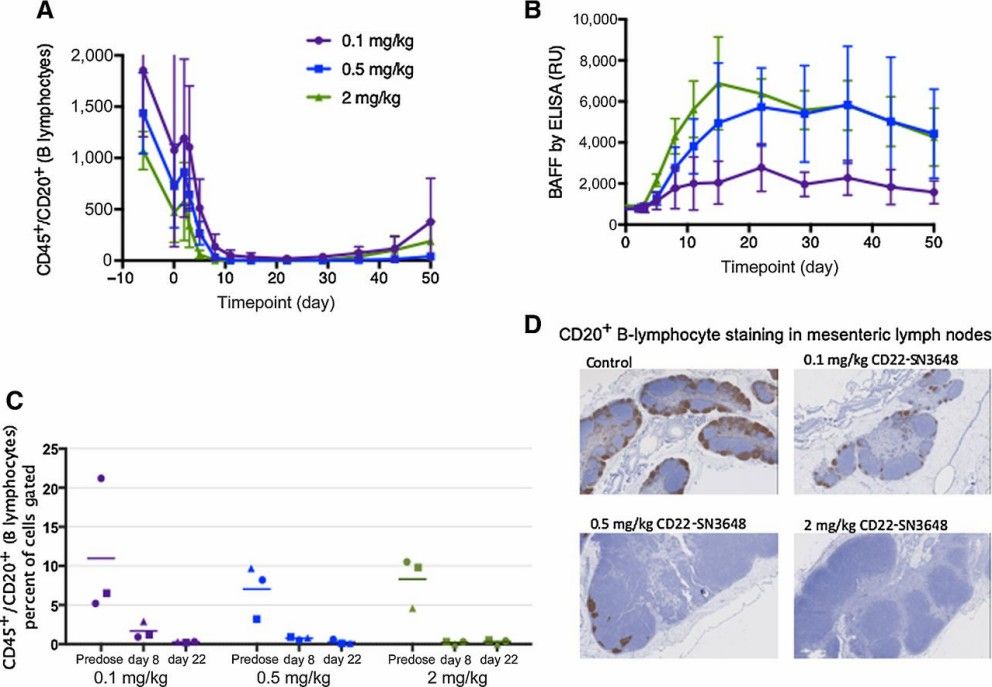 Fig. 5 Time course of peripheral and tissue B-lymphocyte depletion.1
Fig. 5 Time course of peripheral and tissue B-lymphocyte depletion.1
Creative Biolabs has been actively supporting anti-cancer studies for decades and possesses an in-depth understanding of ADC in vivo efficacy. We offer a wide variety of human cancer cell lines for xenograft tumor model development and provide various animal resources to support your study.
Reference
- Yu SF, Lee DW, Zheng B. et al., An Anti-CD22-seco-CBI-Dimer Antibody-Drug Conjugate (ADC) for the Treatment of Non-Hodgkin Lymphoma That Provides a Longer Duration of Response than Auristatin-Based ADCs in Preclinical Models. Mol Cancer Ther. 2021, 20(2): 340-346.
For Research Use Only. NOT FOR CLINICAL USE.

Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
Contact usUSA
Tel:
Fax:
Email:
Europe
Tel:
Email:
Germany
Tel:
Email:

