- Home
- Applications
- Lung Cancer
- Small Cell Lung Cancer
ADC Development Services for Small Cell Lung Cancer (SCLC) Research
The progress made in defining novel therapeutic targets in small cell lung cancer (SCLC) has renewed hope for advances in combatting this recalcitrant disease. Recent pre-clinical and clinical trials results have showed that antibody-drug conjugate (ADC) has great potential in SCLC treatment. Creative Biolabs provides customized service to accelerate the development of the optimal ADC candidate for the target of your interest. Advanced conjugation and linker technologies, associated with a full of payloads endow Creative Biolabs the ability to optimize your ADC right from the outset.
Introduction of SCLC
SCLC is an extremely aggressive cancer that frequently recurs after conventional cytotoxic chemotherapy, accounting for approximately 15% of all lung cancer cases. Among the major lung-cancer subtypes, SCLC has the strongest association with smoking, with only 2% of cases occurring in never-smokers. Consequently, SCLC has a high load of somatic mutations induced by tobacco carcinogens. The most common genetic alterations in SCLC include inactivation of the tumor-suppressor genes TP53 and RB1, as well as copy-number gains of genes encoding MYC family members, enzymes involved in chromatin remodeling, receptor tyrosine kinases and their downstream effectors, and Notch family proteins. Importantly, the high mutational burden of SCLC might provide opportunities for therapeutic intervention.
SCLC has a high propensity to metastasize early during disease development and, at diagnosis, is classified into either limited-stage disease (LD) or extensive-stage disease (ED). Despite high rates of response to first-line chemotherapy and radiotherapy, patients with ED eventually relapse, and very few patients survive more than 5 years from diagnosis.
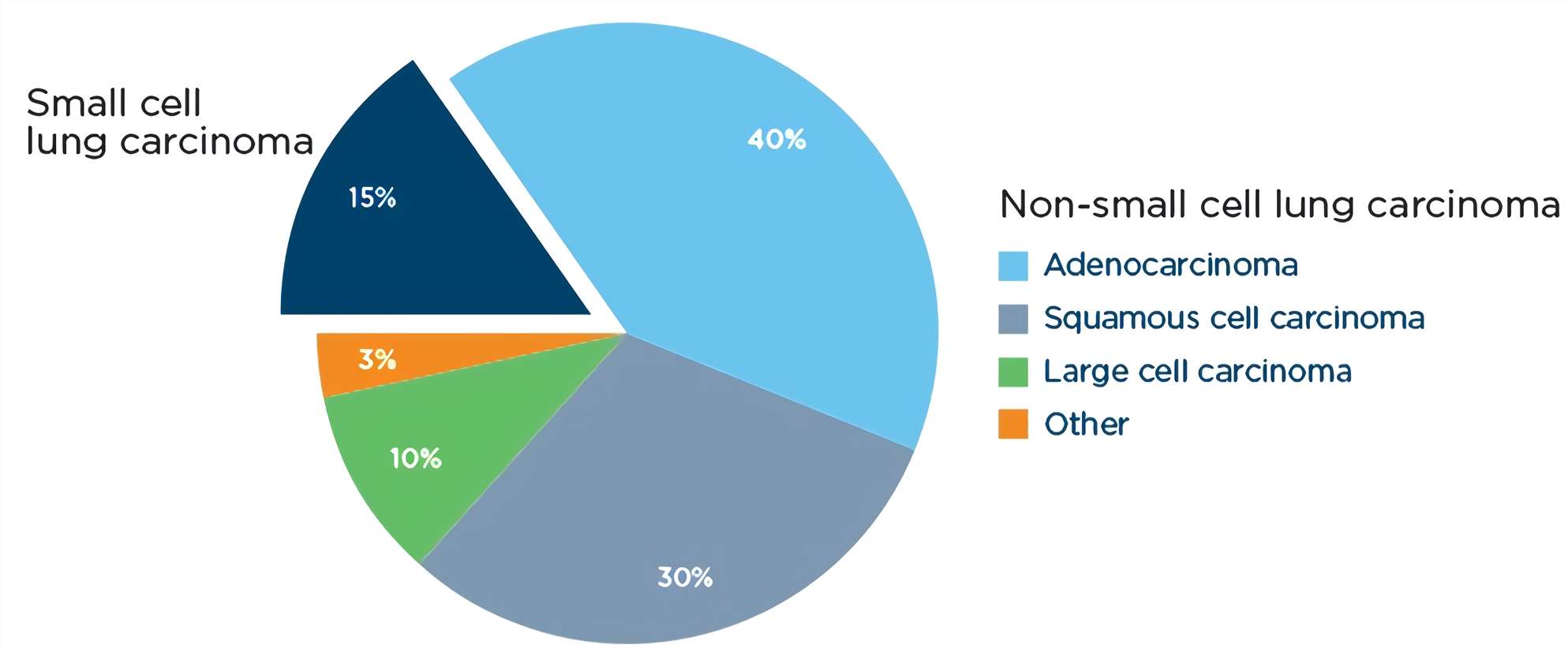 Fig.1 Types of lung cancer by histology.1,3
Fig.1 Types of lung cancer by histology.1,3
SCLC Diagnosis and Treatment
- The diagnosis of SCLC is based primarily on histological appearance by light microscopy, demonstrating dense sheets of small cells with neuroendocrine differentiation (characterized by scant cytoplasm; poorly defined cell borders; dispersed, finely granular nuclear chromatin; absent or inconspicuous nucleoli; and prominent nuclear moulding).
- Treatment most often involves platinum-based combination chemotherapy, hyperfractionated thoracic radiation, and prophylactic cranial irradiation. However, the standard-of-care chemotherapy involves combination of a platinum agent with etoposide, and efforts to improve on the activity of this regimen by including a third agent in the combination have been largely unsuccessful. Thoracic radiotherapy plays an indispensable role in the treatment of LD-SCLC. Etoposide in combination with a platinum agent (carboplatin or cisplatin) remains the standard first-line option for ED-SCLC patients. While addition of an anti-PD-L1 antibody to first-line treatment may benefit many patients, it may limit the development and use of anti-PD-1(L1) agents in second or later lines. The approval of atezolizumab and subsequent changes to the standard of care may also result in challenges to the conduct and interpretation of ongoing clinical trials in the first-line and the maintenance settings. Treatment options for patients in the second line and beyond remain limited, highlighting the need for development of additional therapies.
Targeting Specific Surface Markers
While conventional chemotherapy is directed against all rapidly dividing cells, targeted therapies focus on either the tumor cells or the peritumoral environment. Hereby, targeted therapies either interact with key tumor drivers that promote cell growth as well as immortality or address tumor-specific intra- or extracellular features. SCLC tissues very likely reveal specific surface markers, and these are useful factors for specifically directing cytotoxic agents to SCLC tissues. For instance, rovalpituzumab tesirine is an anti-DLL3 ADC, the activity of which is being investigated in a third-line patient population, a patient population without any approved therapies. Figure 3 depicts relevant antibody-drug/cytokine conjugates tested as new therapeutic constructs for SCLC. Creative Biolabs evaluated current literature for potential new surface markers and identified several promising markers for ADC development targeting SCLC.
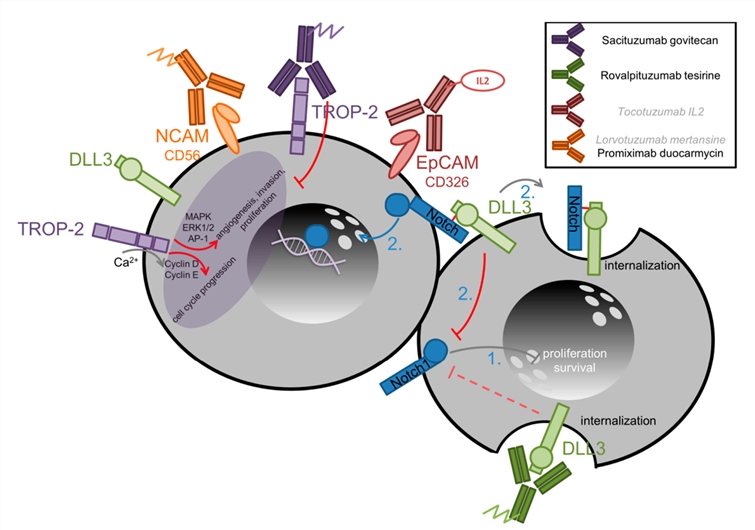 Fig.2 Targeting surface markers and TROP-2 and DLL3 mechanisms of action in SCLC.2,4
Fig.2 Targeting surface markers and TROP-2 and DLL3 mechanisms of action in SCLC.2,4
Our Featured ADC Development Services
Creative Biolabs can help clients make informed decisions on all components of the ADC structure so that the optimal candidate is identified for your target of interest in terms of stability, off-target toxicity and efficacy. Additionally, once the optimized ADC has been identified, the chosen candidate can be seamlessly transferred into Creative Biolabs’s manufacturing stream, allowing the accelerated production of different scale according to your requirement.
- Benefit from access to Creative Biolabs’ wide range of expertise and state-of-the-art facilities to streamline your ADC development program
- Comprehensive service offering for all ADC components
- Generate mutiple potential candidates for assessment before committing to further development
If you are interested in our anti-SCLC ADC development services, please don’t hesitate to contact us for more information and a detailed quote.
References
- Ahmed, Bakhan Tofiq. "Lung cancer prediction and detection using image processing mechanisms: an overview." Signal and Image Processing Letters 1.3 (2019): 20-31.
- Schulze, Arik Bernard, et al. "Future options of molecular-targeted therapy in small cell lung cancer." Cancers 11.5 (2019): 690.
- Distributed under Open Access License CC BY SA 4.0, without modification.
- Distributed under Open Access License CC BY 4.0, without modification.
For Research Use Only. NOT FOR CLINICAL USE.

Online Inquiry
Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
