- Home
- Resources
- Knowledge Center
- Reviews
- Advancements in Antibody-Drug Conjugate Technologies
Advancements in Antibody-Drug Conjugate Technologies
Antibody-drug conjugates (ADCs), a new drug for cancer treatment, combine the targeting of monoclonal antibodies with the efficacy of small molecules. Since the U.S. Food and Drug Administration (FDA) pioneered ADC therapeutics through its first approval in 2009, this field has undergone transformative evolution. Over two decades of iterative innovation, ADC development has progressed through three distinct generations: First-generation constructs established fundamental architecture, while second-generation agents demonstrated enhanced therapeutic efficacy through improved antigen specificity. The current third-generation ADCs address prior limitations in payload delivery and off-target effects, achieving optimized therapeutic indices through refined tumor targeting, enhanced cytotoxic payload release mechanisms, and expanded clinical versatility. This explosive advancement is further propelled by the identification of novel tumor-specific antigen targets and breakthroughs in intratumoral drug activation paradigms. With over 15 FDA-approved ADCs and 100+ candidates in clinical pipelines, the field now enters a golden era of precision oncology, revolutionizing therapeutic strategies across hematological and solid malignancies while redefining standards for targeted cancer therapeutics.
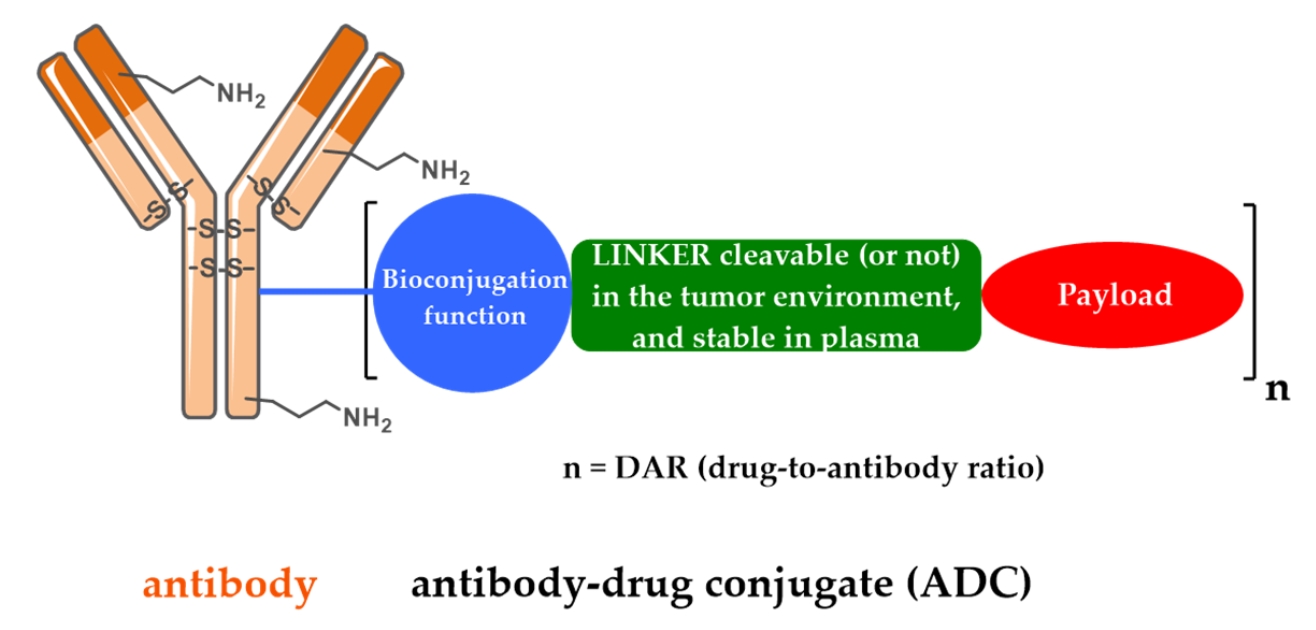 Fig. 1 Schematic representation of the first generation FDA-approved ADCs.1
Fig. 1 Schematic representation of the first generation FDA-approved ADCs.1
First-Generation ADCs: Foundations and Limitations
The first ADCs solidified the concept of targeted chemotherapy through the use of recombinant humanized IgG4 kappa antibodies against CD33-positive cells. The designs used hydrazone linkers for the attachment of cytotoxic payloads, leveraging the acidic tumor microenvironment to induce pH-dependent release of the drug. Binding to the target initiated the process of receptor-mediated internalization of the antibody-CD33 complex, during which intracellular release of very potent DNA-damaging molecules such as calicheamicin derivatives or anthracyclines occurred. While this generation delivered proof-of-concept for ADC mechanisms, its therapeutic index was constrained by fundamental deficiencies: stochastic conjugation chemistry created heterogeneous drug-to-antibody ratios (DARs) with large unconjugated antibody populations that competed for target binding. Furthermore, acid-labile linker technology exhibited less-than-optimal stability in systemic circulation, resulting in premature payload release and off-target toxicity. These early difficulties motivated subsequent innovations in ADC engineering, most prominently in linker chemistry and site-specific conjugation platforms.
First-generation ADCs were faced with a number of primary limitations that mitigated therapeutic efficiency. To begin with, low drug potency accompanied by subtherapeutic plasma concentrations and low expression of target antigens resulted in insufficient intracellular payload accumulation, falling short of tumoricidal values. Two fundamental challenges emerged: payload pharmacology constraints, including DNA-damaging agents such as calicheamicin lacking sufficient bystander effects to overcome antigen heterogeneity, and biological constraints such as limited tumor penetration and high-rate efflux mechanisms. Second, chemically labile linkers had premature cleavage in vivo, resulting in off-target payload release and dose-limiting systemic toxicities—illustrated by the hepatotoxicity encountered in early anti-CD33 conjugates. Third, therapeutic use was restricted to hematological malignancies due to dependence upon highly internalizing antigens like CD33, while solid tumors remained refractory due to stromal barriers, antigen downregulation, and lysosomal trafficking deficiencies. Finally, mechanisms of acquired resistance like antigen escape variants and lysosomal enzyme polymorphisms further devalued clinical usefulness. All these shortcomings cumulatively underscored the need for next-generation innovations in payload design, linker stability, and modulation of tumor microenvironment.
Second-Generation ADCs: Enhanced Stability and Payload Diversity
Second-generation ADCs illustrated notable therapeutic advances by overcoming chief frailties of their native counterparts. Some major improvements were as follows:
Antibody engineering by embracing chimeric and humanized monoclonal antibodies in place of murine-derived counterparts to reduce immunogenicity;
Linker stabilized through protease-cleavable mechanism in conjunction with self-immolative spacers to ensure targeted release of the payload in lysosomal environments and circulatory integrity; Improved cytotoxicity through newer payloads like microtubule-disrupting agents DM1 and MMAE possessing subnanomolar activity. These innovations complementarily expanded therapeutic windows, allowing effective targeting of both solid tumors and hematologic malignancies. Of special note was the combination with microtubule inhibitors, which added new mechanisms of action—mitotic arrest and immunogenic cell death—while new engineered conjugation strategies allowed more consistent drug-to-antibody ratios. Second-generation constructs extended these advances with better tumor penetration and bystander effects, overcoming antigen heterogeneity and stromal barriers that had blunted solid tumor efficacy. This paradigm establishment lay down foundational bases for modern ADC optimization techniques.
Despite notable progress, second-generation ADCs retain inherent limitations in their molecular construct. Inhomogeneous drug-to-antibody ratios persist, with a significant proportion of unconjugated "naked" antibodies in the bloodstream. The naked antibodies compete with their drug-conjugated equivalents for antigen-binding sites, lowering therapeutic activity. Concurrently, overconjugation of cytotoxic payloads results in antibody aggregation, increases systemic clearance, and amplifies off-target toxicity due to payload release nonspecificity. Further, low antigen-density tumors or microenvironments rich in stroma display suboptimal reactivity, highlighting the demand for new approaches to targeting and overcoming these biological challenges.
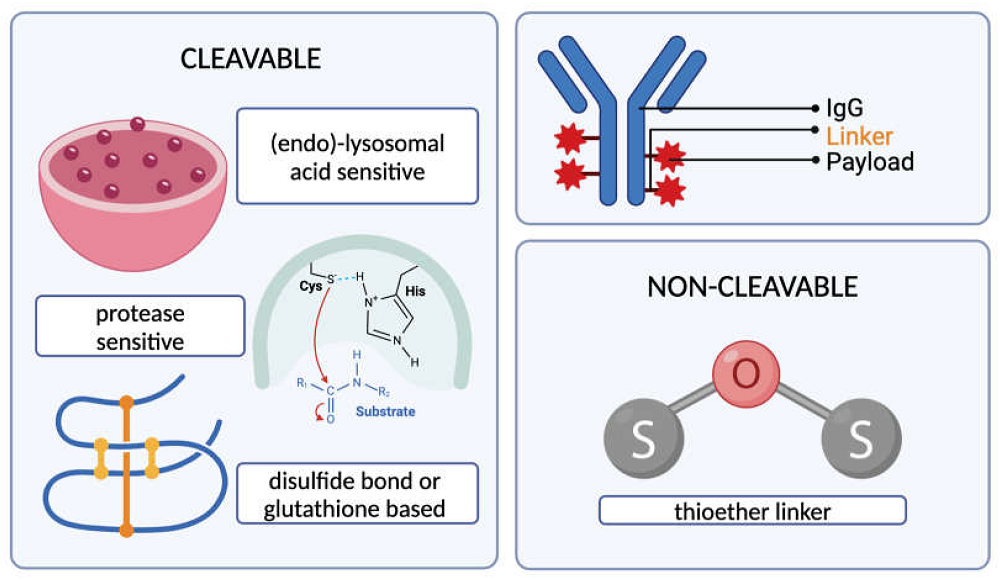 Fig. 2 Types of cleavable and non-cleavable ADC linkers.4
Fig. 2 Types of cleavable and non-cleavable ADC linkers.4
Together, the second-generation site-specific conjugation technologies enable the attachment of defined bioorthogonal moieties onto antibodies for targeted payload attachment at designed sites. Introduction of such functional motifs, however, requires some level of structural modification of the antibody scaffold that may have unpredictable effects on its conformational stability, folding kinetics, or antigen-binding affinity, posing a significant challenge to further downstream manufacturing process, including optimizing batch-to-batch consistency, reducing aggregation, and evaluating functionality of the engineered constructs.
Third-Generation ADCs: Precision Engineering and Novel Mechanisms
During the generation of a new generation of ADC drugs, it is essential to clarify some salient points. The therapeutic efficacy of ADCs relies on two basic requirements. Firstly, a strong chemical bridge between the cytotoxic payload and antibody is necessary. Such a linker should provide outstanding stability for systemic circulation so that off-target release of the payload is avoided, yet concurrently show prompt responsiveness to lysosomal environments following internalization. Such dual functionality enables tumor-selective payload delivery while minimizing systemic toxicity. Second, the payload itself must possess ultrahigh potency to overcome biological barriers such as low antigen density and heterogeneous drug distribution. Early-generation ADCs utilizing moderately potent agents like anthracyclines suffered from narrow therapeutic indices, wherein maximum tolerated doses (MTD) failed to achieve clinically meaningful tumor regression due to sublethal intratumoral drug concentrations.
The current generation emphasizes site-specific conjugation, novel payload classes, and innovative targeting paradigms. Engineered cysteine residues or enzymatic methods enable homogeneous DARs, enhancing pharmacokinetic predictability. For instance, enzymatically cleavable tetrapeptide linkers paired with hydrophilic spacers reduce aggregation while improving tumor-selective payload release.
Third-generation ADCs also explore non-internalizing antigens, leveraging extracellular proteases or reducing environments for payload activation. For example, conjugates targeting tumor-associated extracellular matrix components exploit stromal proteases to release potent DNA crosslinkers, bypassing antigen internalization requirements. Additionally, novel payloads such as RNA polymerase II inhibitors or topoisomerase I inhibitors demonstrate efficacy against quiescent or multidrug-resistant cells.
Antibody fragments and biparatopic designs enhance tumor penetration and antigen engagement. These smaller formats improve diffusion through dense stroma while maintaining high affinity. Furthermore, "bystander effects" enabled by membrane-permeable payloads—are harnessed to eradicate antigen-negative neighboring cells, addressing intratumoral heterogeneity.
Third-Generation ADC Platforms
Third-generation ADCs leverage advanced engineering to address limitations of earlier designs. Bispecific ADCs bind two antigens or epitopes to address tumor homogeneity and drug resistance, enhancing internalization and payload delivery through effects such as receptor aggregation. Continuously active ADCs, such as prodrug conjugates, have TME-responsive elements that minimize off-target toxicity while achieving local activation. Immunostimulatory ADCs combine antibodies with immunomodulatory drugs to trigger innate and adaptive immunity by targeting delivery of antigen presenting cells. The protein degrader ADC uses protein decomposition targeting inlay to ubiquitously proteinize and degrade cancer proteins, expanding treatment pathways for "non-treatable" targets. Dual-drug ADCs use a vertical binding strategy to combine microtubule inhibitors and DNA damaging agents to provide synergistic activity and drug resistance in heterologous tumors. These platforms emphasize accuracy, low toxicity and multimodal action to maximize therapeutic indicators and combat the complex biology of cancer.
Expanding Therapeutic Horizons
Recent innovations extend ADC applications beyond cancer. Immunomodulatory conjugates such as glucocorticoid-conjugated anti-macrophage-specific receptor antibodies direct anti-inflammatory action with less systemic toxicity. Similarly, antibody-antibiotic conjugates deliver the potent antimicrobial agent intracellularly to kill pathogens shielded within host cells, offering treatment for antibiotic-resistant infections.
Future Directions in ADC Development
The evolution of ADCs will focus on addressing unresolved challenges and expanding therapeutic potential.
Precision Targeting and Biomarker Identification. Patient stratification using predictive biomarkers will optimize ADC efficacy. Biomarker-driven trials are critical for identifying responders to bispecific or immune-stimulating ADCs, particularly in heterogeneous tumors.
Linker and Payload Innovation. Next-generation linkers will balance stability and controlled payload release, minimizing premature toxicity. Hydrophilic modifications and enzyme-cleavable designs aim to improve pharmacokinetics. Novel payloads, including RNA polymerase inhibitors and molecular glues, will diversify mechanisms of action.
Multimodal Integration. ADCs in combination with immunotherapy, radiotherapy, or targeted therapies can augment antitumor activities. For instance, ISACs with checkpoint inhibitors can further increase T-cell activation, while DACs can be combined with kinase inhibitors in resistant tumors.
Enhanced Homogeneity and Site-Specific Conjugation. Advances in enzymatic and chemical conjugation will yield homogeneous ADCs with defined DARs, improving therapeutic windows.
Toxicology Mitigation. Strategies to reduce hepatic uptake, ocular toxicity, and thrombocytopenia—common with hydrophobic payloads—include structural optimization of antibodies and payload-linker complexes.
In conclusion, third-generation ADCs exemplify the convergence of molecular engineering and tumor biology. By refining specificity, payload diversity, and combinatorial approaches, future ADCs hold promise for transforming outcomes in refractory cancers while minimizing adverse effects.
Future Perspectives
ADC technology continues to evolve, driven by advances in protein engineering, linker chemistry, and mechanistic understanding. Challenges such as stromal barriers, resistance pathways, and optimal combination therapies remain focal points. Emerging trends include dual-payload systems, conditionally active prodrugs, and integration with immune checkpoint modulation. By marrying precision targeting with adaptable payload strategies, next-generation ADCs hold promise as truly modular therapeutics, transcending traditional oncology to address diverse diseases.
As a leading ADC developer, Creative Biolabs offers end-to-end solutions to accelerate antibody-drug conjugate R&D. Our one-stop ADC service spans three key areas:
Antibody Discovery: Use phage display and humanization tech to identify high-specificity antibodies, validated via our rapid "Anti-Ab ADCs" system.
Core Component Engineering:
- DrugLnk™ Synthesis: Design custom linkers/payloads (e.g., cleavable linkers, DNA-damaging agents) for diverse mechanisms.
- Antibody Conjugation: Precision DAR control via cysteine modification ensures stability and reduces toxicity.
Comprehensive Analysis:
- In Vitro: Assess purity, DAR, cytotoxicity, and 3D tumor penetration.
- In Vivo: Evaluate PK/PD and safety for IND readiness.
With 10+ years of expertise and customizable modules, we streamline workflows to fit budgets/timelines. From antibody discovery to preclinical candidates, trust us to drive your ADC project forward. Contact for a tailored quote!
References
- Joubert, N.; Beck, A.; Dumontet, C.; Denevault-Sabourin, C. Antibody–Drug Conjugates: The Last Decade. Pharmaceuticals 2020, 13, 245. https://doi.org/10.3390/ph13090245
- Fu, Z.; Li, S.; Han, S. et al. Antibody drug conjugate: the "biological missile" for targeted cancer therapy. Sig Transduct Target Ther 2022, 93, 7. https://doi.org/10.1038/s41392-022-00947-7
- Fujii, T.; Matsuda, Y.; Seki, T.; et al. AJICAP second generation: improved chemical site-specific conjugation technology for antibody–drug conjugate production. Bioconjugate Chemistry 2023, 34(4): 728-738. https://doi.org/10.1021/acs.bioconjchem.3c00040
- Buyukgolcigezli, I.; Tenekeci, A.K.; Sahin, I.H. Opportunities and Challenges in Antibody–Drug Conjugates for Cancer Therapy: A New Era for Cancer Treatment. Cancers 2025, 17, 958. https://doi.org/10.3390/cancers17060958
For Research Use Only. NOT FOR CLINICAL USE.

Online Inquiry
Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
