- Home
- Resources
- Knowledge Center
- Antibody–drug Conjugate: Highly Targeted Biopharmaceutical Drug
Antibody–drug Conjugate: Highly Targeted Biopharmaceutical Drug
What is ADC
As antibody technology has gradually improved over the past few decades, antibody-conjugated drugs have also entered a period of rapid development. ADCs are highly targeted biopharmaceuticals. It combines monoclonal antibodies specific for surface antigens present on specific tumor cells with highly effective anti-cancer drugs linked through chemical linkers. ADCs are made up of monoclonal antibodies coupled to different numbers of small molecule cytotoxins through linkers. Monoclonal antibodies' high-affinity antigen recognition domains enable clathrin-dependent receptor-mediated endocytosis of ADC complexes. Consequently, the therapeutic efficacy of ADCs is principally determined by two sequential biological processes: target-specific antigen-antibody interaction governed by binding avidity and epitope accessibility, and antigen density-dependent internalization kinetics regulating intracellular payload release. This dual-targeting mechanism endows ADCs with superior tumor selectivity compared to conventional chemotherapeutic agents, establishing them as precision-guided therapeutic systems in modern oncology. In simple terms, ADC, due to its high targeting specificity compared to traditional drugs, is also referred to as a "biological missile".
ADC Construction
The components of an ADC are intricately linked to its highly targeted function. Alterations in each element can impact the final efficacy and safety of the ADC. To achieve the desired level of an ADC drug, which remains stable in the bloodstream, accurately reaches the therapeutic target, and ultimately releases the cytotoxic payload, the development of an ADC must consider all these critical components, including the target antigen, antibody, cytotoxic payload, linker, and the conjugation method.
| Construction | Details |
| Monoclonal Antibody | This highly targeted monoclonal antibody selectively attaches to antigens that identify either malignant cells or tumor-specific markers appearing on malignant cell surfaces. The antibodies demonstrate engineered properties for superior antigen binding capacity and internalization performance while maintaining compatibility with the linker chemistry. |
| Linker | Linker A covalent bridge that either remains chemically stable or can be cleaved to bind an antibody to its cytotoxic payload. Linkers maintain stability throughout systemic circulation until they release the payload specifically in the tumor microenvironment through enzymatic cleavage, acidic pH conditions, or reducing environments. |
| Payload | The cytotoxic payload, which includes drugs like microtubule inhibitors as well as DNA alkylators and topoisomerase inhibitors or emerging treatments such as immune stimulators and protein degraders, eliminates cancer cells when released inside cells. |
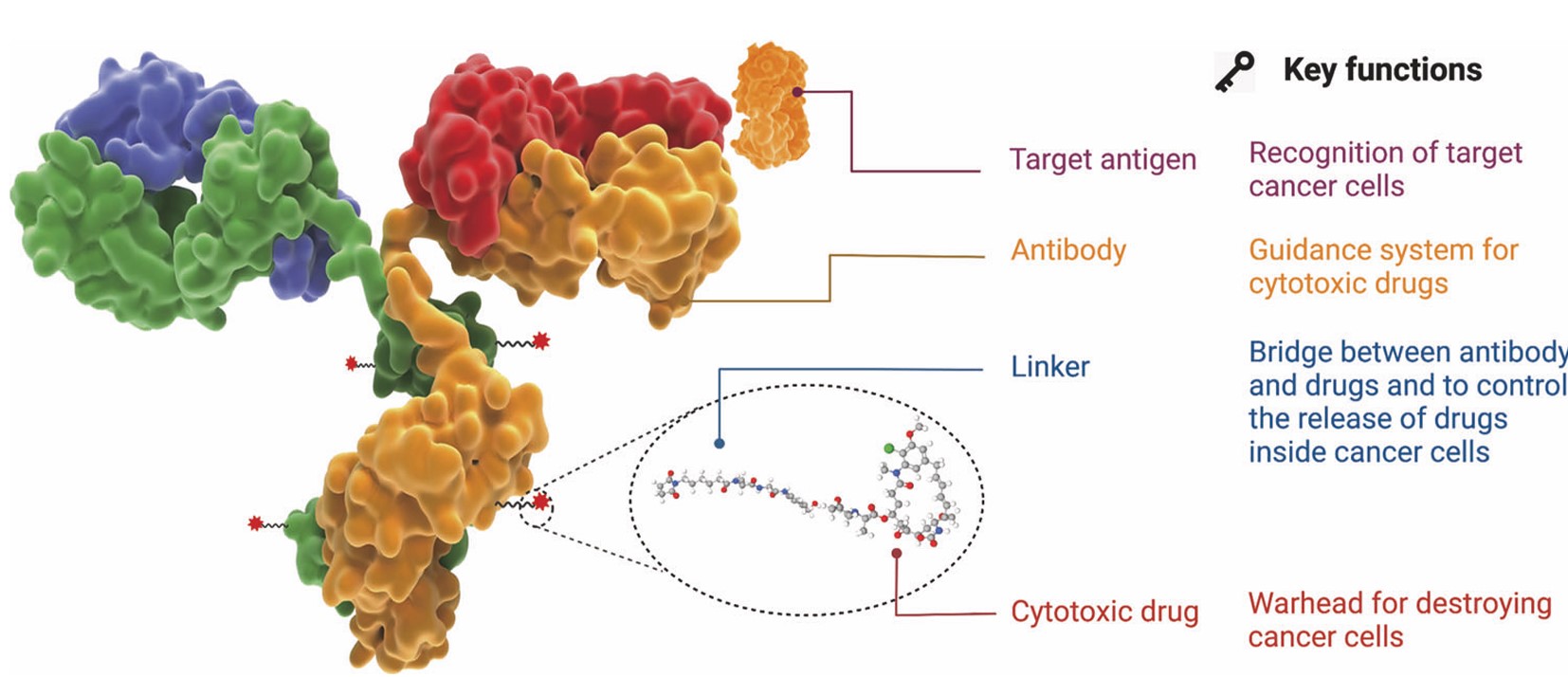 Fig. 1 The structure and characteristic of an ADC drug.1,5
Fig. 1 The structure and characteristic of an ADC drug.1,5
Antibody Selection
The antibody component functions as the molecular guidance system of ADCs, mediating tumor-specific cytotoxicity through high-affinity binding to tumor-associated surface antigens. This targeted payload delivery mechanism is fundamentally dependent on the antibody's ability to establish precise antigen recognition, thereby serving as the critical determinant for establishing specific molecular interactions between the therapeutic agent and its designated cellular target.
As crucial antigen recognition elements, these antibodies must demonstrate subnanomolar binding affinity coupled with efficient receptor-mediated endocytic trafficking. Optimal antibody engineering necessitates three core pharmacological attributes, rapid clathrin-dependent internalization kinetics, minimal immunogenicity through humanization, and extended plasma persistence via FcRn recycling.
Currently, all ADCs in clinical and preclinical development are derived from the four subclasses of IgG (Fig. 2). These four subclasses have approximately 90% sequence homology, but may differ in other aspects. For example, their serum stability, number of interchain disulfide bonds, and ability to activate the immune system through antibody dependent cytotoxicity or complement pathways differ. Consequently, ADC development requires researchers to choose particular subclasses of antibodies according to specific therapeutic needs.
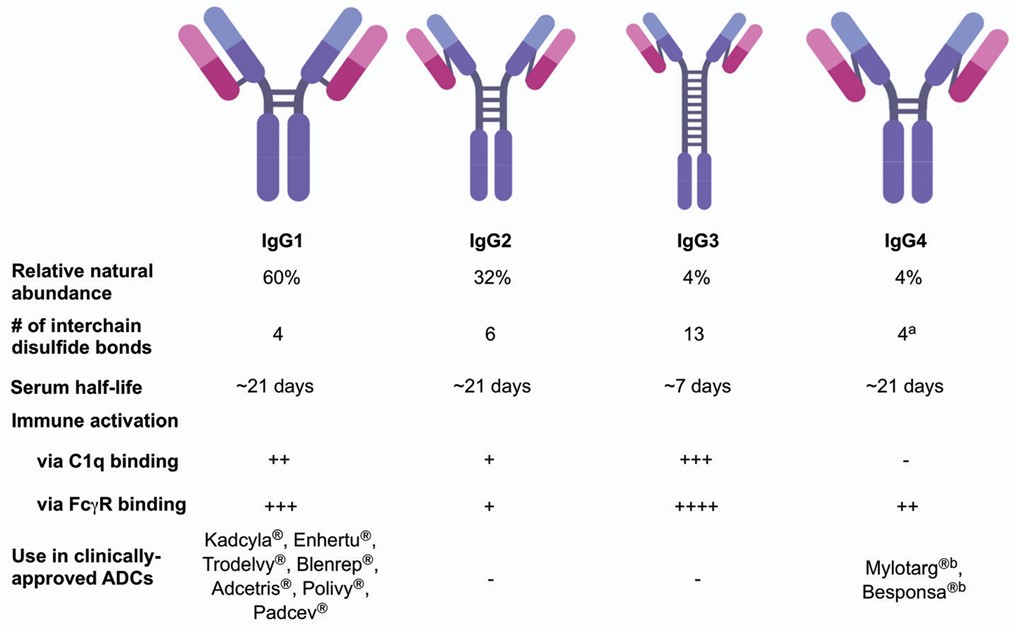 Fig. 2 Overview of IgG subclasses. 3
Fig. 2 Overview of IgG subclasses. 3
In addition to demonstrating high binding affinity to the target antigen, an ideal antibody component should contribute to effective internalization. The high affinity of antibodies facilitates efficient endocytosis of ADC drugs. Enhanced endocytic efficiency reduces off-target action of ADC medicines, thus augmenting therapeutic activity and safety profiles. Additionally, certain antibodies exhibit inherent therapeutic activity that synergistically enhances cytotoxic activities of ADC drugs against target cells.
Linkers
The linker constitutes a critical structural element connecting the monoclonal antibody to its cytotoxic payload. Its dual functionality ensures payload retention during systemic circulation while enabling site-specific release within tumor microenvironments. Crucially, linker chemistry profoundly influences ADC stability, with pharmacokinetic and pharmacodynamic properties being dictated by both linker specificity and conjugation site selection between the antibody and payload.
Current FDA-approved antibody-drug conjugates primarily employ two distinct conjugation strategies, including cleavable linkers, and non-cleavable linkers (a non-cleavable thioether linker that dispenses the medical preparation after the monoclonal antibody has dissipated).
Explore our extensive collection of ADC linker products and discover new favorites:
| Catalog | Product name | CAS NO | Molecular Weight | Inquiry |
ADC-L-003 
|
Fmoc-Val-Cit-PAB-OH | 159858-22-7 | 601.7 | Inquiry |
ADC-L-004 
|
Fmoc-Val-Cit-PAB-PNP | 863971-53-3 | 766.8 | Inquiry |
ADC-L-007 
|
Val-Cit-PAB | 159857-79-1 | 379.45 | Inquiry |
ADC-L-008 
|
MC-Val-Cit-PAB-PNP | 159857-81-5 | 737.77 | Inquiry |
ADC-L-016 
|
SMCC | 64987-85-5 | 334.32 | Inquiry |
| ADC-L-001 | (Ac)Phe-Lys(Alloc)-PABC-PNP | 689.71 | Inquiry | |
| ADC-L-002 | Mal-heptanoic NHS ester | 55750-63-5 | 308.3 | Inquiry |
| ADC-L-006 | Val-cit-PAB-OH | 159857-79-1 | 379.45 | Inquiry |
| ADC-L-009 | Phe-Lys(Trt)-PAB | 1116085-99-4 | 640.81 | Inquiry |
Cytotoxic Payloads
The cytotoxic payload is the pharmacologically active moiety of the drug in ADCs, acting as the therapeutic warhead that forms highly active antitumor activity through hydrolytic cleavage. Clinical development in anticancer ADCs continues to focus intensively on two classes of cytotoxic agents: DNA-damaging agents and tubulin polymerization inhibitors. Besides native cytotoxicity, payload candidates also need to possess appealing conjugation compatibility, solubility, and stability profiles to fulfill ADC engineering requirements.
Mechanism of Action of ADC
The selectivity of ADC targeting is predominantly regulated by their mAb moiety. Figure 3 illustrates the general mechanism, where the primary process involves antigen-mediated internalization of the ADC complex into the tumor cells and subsequent cleavage of the linker to enable intracellular release of the cytotoxic payload. This payload causes therapeutic effects through microtubule-disrupting or DNA-damaging mechanisms. Permeable payloads to membranes can also induce bystander effects by transmembrane diffusion, enhancing therapeutic efficacy by affecting adjacent malignant cells. Notably, bystander activity in this context can modify tumor microenvironment conditions, with a potential to trigger a feedforward loop to enhance ADC cytotoxicity. Antibody-antigen binding and subsequent internalization processes may have to be pharmacologically optimized to maximize therapeutic benefit.
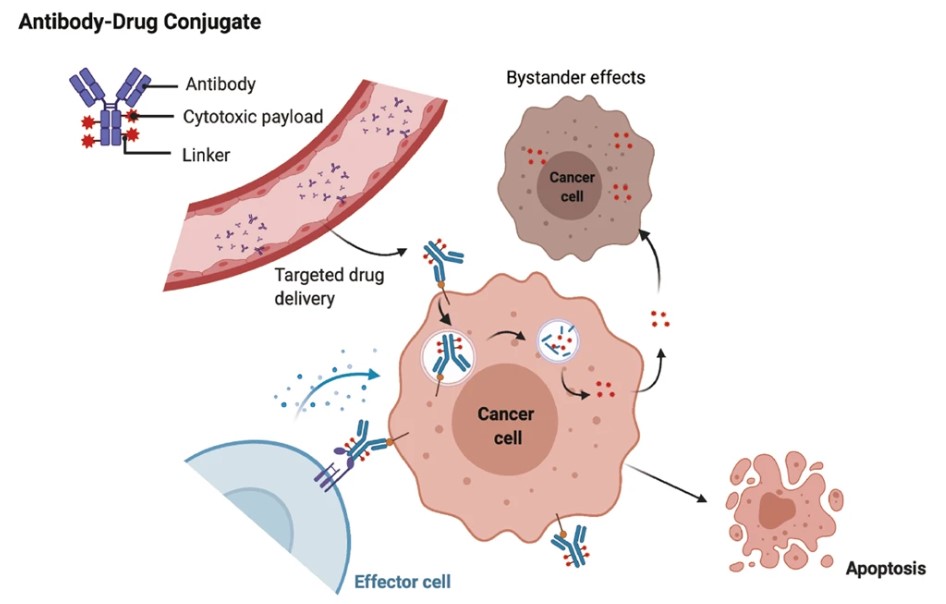 Fig. 3 The main core mechanism of action of ADCs. 1,5
Fig. 3 The main core mechanism of action of ADCs. 1,5
ADCs for Cancer Therapy
Cancer is the second leading worldwide health hazard. Conventional chemotherapeutic approaches, which have employed cytotoxic drugs mainly, have been the mainstay of therapy for an extensive variety of malignancies. Representative categories are DNA base analogs, DNA-interacting compounds, and antimetabolites. But they are not specific for biodistribution and typically cause off-target tissue exposure, resulting in severe side effects and therefore low therapeutic indices. The advent of ADCs revolutionized cancer treatment modalities, fulfilling Paul Ehrlich's visionary concept of "magic bullets" to specifically kill cancer cells with an intact normal tissue.
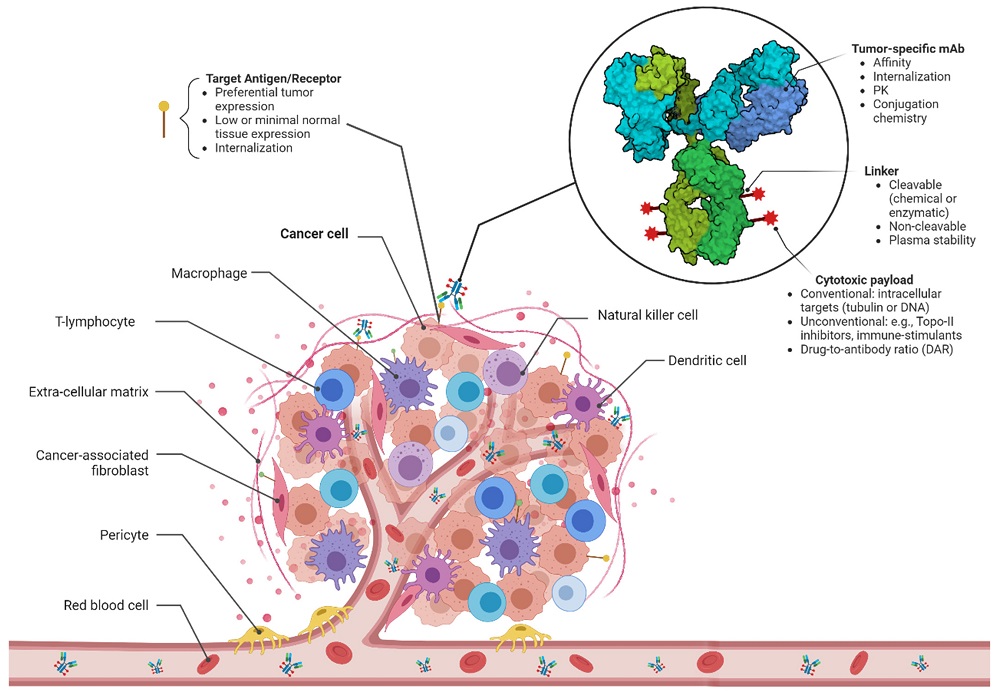 Fig. 4 ADCs for cancer therapy. 2,5
Fig. 4 ADCs for cancer therapy. 2,5
Unlike mAb monotherapy, ADCs were structured to synergistically combine the targeting specificity of monoclonal antibodies and the chemotherapeutic drug's cytotoxicity as an effort to ensure maximal therapeutic windows. These compound forms of treatment have become an interesting field to focus on for oncology drug development since they now have the ability to contribute tumor-targeting specificity by mAbs and cytotoxic effects confined to malignant tissue at the same time. One of the fastest-growing cancer precision medicine therapy areas is represented by ADCs, which represent the best possible combination of biologics and small-molecule drugs to achieve spatial site-specific ablation.
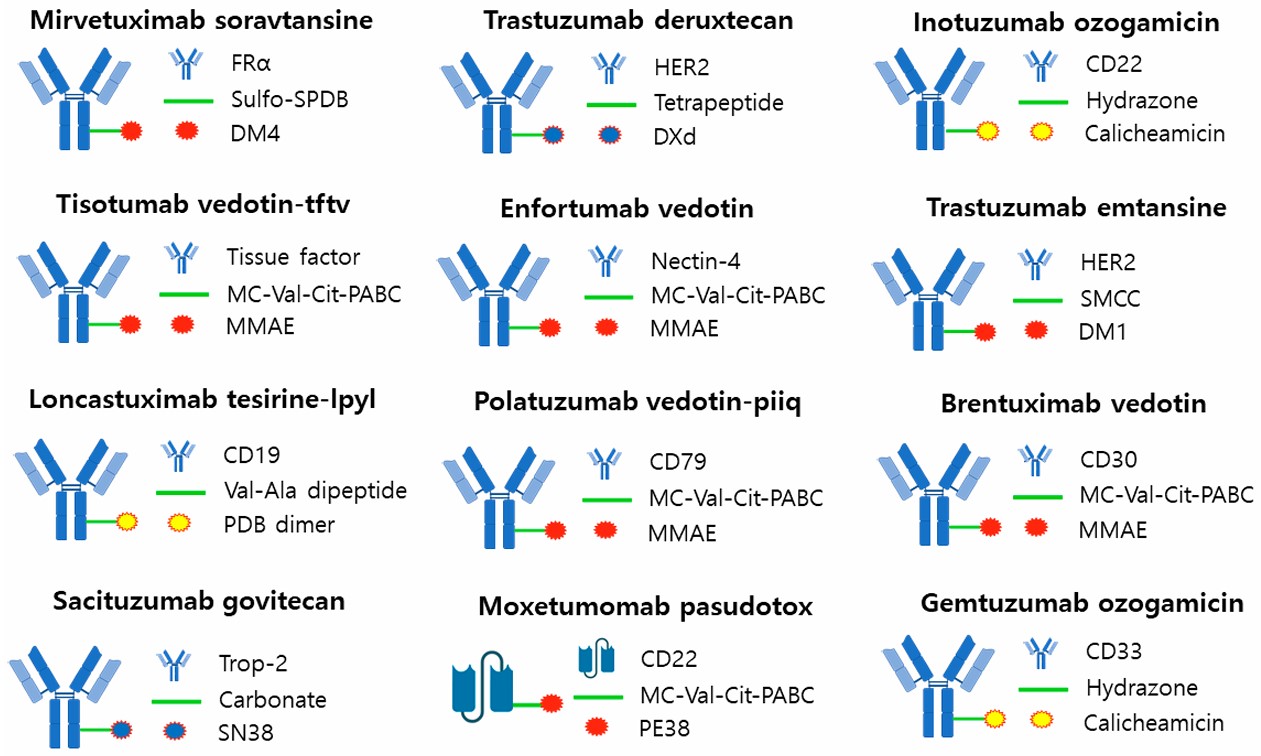 Fig. 5 FDA-approved ADC. 4,5
Fig. 5 FDA-approved ADC. 4,5
Currently, over 100 ADC candidates are undergoing various stages of clinical evaluation, with 12 therapeutic agents already approved for clinical use. The arrival of ADC drugs has provided new hope to cancer treatment, so that the treatment of cancer is no longer limited to chemotherapy drugs. Of ADC drugs, they have a certain effect on the treatment of some cancers. Brentuximab ab vedotin has an effect in the treatment of hematological malignancies, ado-trastuzumab emtansine has an effect in the targeting treatment of HER2-positive metastatic cancers, and Sacituzumab govitecan has an effect in the treatment of triple negative breast cancer.
History of ADC Development
The initial approved ADC, gemtuzumab ozogamicin, was for AML treatment in 2000. Although early ADCs have shown efficacy for the treatment of AML, they are also beset by instability, immunogenicity and off-target toxicity, such that clinical successes have been modest. It was not until 2011 that Brentuximab vedotin and ado-trastuzumab emtansine were approved as second-generation ADCs. Their success in Hodgkin lymphoma and HER2-positive breast cancer, respectively, confirmed enhanced joint stability and payload delivery. A total of 14 ADCs were FDA-approved as of 2024, and over 200 drug candidates are in clinical development.
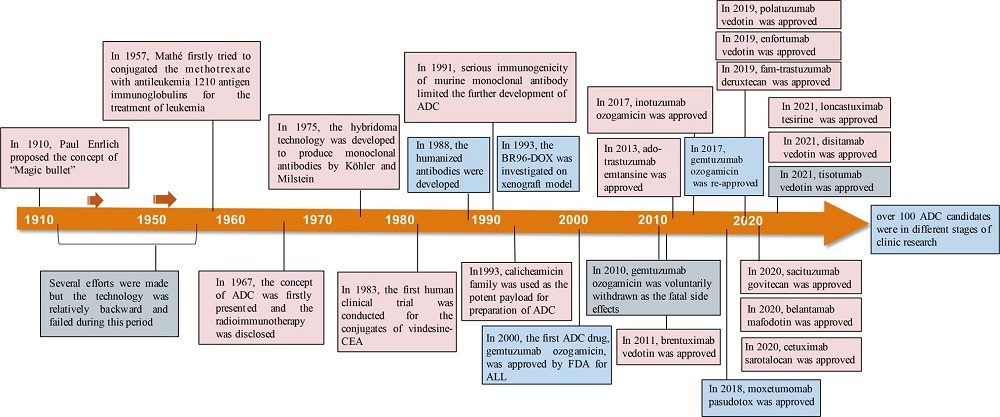 Fig. 6 Timeline depicting important events in the development and approval of ADC drugs. 1,5
Fig. 6 Timeline depicting important events in the development and approval of ADC drugs. 1,5
Clinical Impact and Challenges of ADC
ADC drugs have changed the treatment paradigm for hematological and solid malignancies. For example, trastuzumab deruxtecan has shown unprecedented efficacy in low HER2 breast cancer, while saxizumab gove can improve the survival rate of cancer triple negative breast cancer. However, challenges persist:
Toxicity: Dose-limiting adverse events, such as interstitial lung disease (ILD) and thrombocytopenia, often stem from payload release in normal tissues or Fc-mediated uptake.
Resistance: Mechanisms include antigen downregulation, defective internalization, and drug efflux pumps.
Heterogeneity: Tumor antigen variability and stromal barriers limit ADC penetration, particularly in solid tumors.
Future Directions of ADC
Next-generation ADCs are overcoming current treatment limitations by employing three strategic approaches. First, novel targets such as tumor microenvironment antigens and dual-targeting approaches are being explored to reduce mechanisms of resistance. Second, non-internalizing ADC platforms deliver their drugs outside the cell via enzyme-activated linkers to bypass the need for repeated antigen internalization. Third, synergistic combinations with immune checkpoint inhibitors or PARP inhibitors are also under investigation to enhance antitumor activity and treat resistance. These advances collectively serve to improve tumor specificity and therapy efficacy.
Creative Biolabs offers comprehensive services for Antibody-Drug Conjugate (ADC) development, covering antibody discovery targeting tumor surface and microenvironment antigens. Our DrugLnk™ platform provides custom synthesis of drug and linker modules. We offer diverse antibody conjugation strategies, including lysine, cysteine, tyrosine, enzyme-mediated, and carbohydrate-based methods. Creative Biolabs provides robust in vitro analysis, including ADC biochemical characterization, efficacy evaluation, and advanced 3D cell culture models. Our in vivo services encompass pharmacokinetics, safety assessment, efficacy evaluation in animal models, and ADC immunogenicity analysis. Additionally, we offer streamlined ADC manufacturing services. Creative Biolabs aims to be a one-stop solution for accelerating ADC development programs.
References
- Fu Z, Li S, Han S, et al. Antibody drug conjugate: the "biological missile" for targeted cancer therapy. Sig Transduct Target Ther 2022,7,93. https://doi.org/10.1038/s41392-022-00947-7
- Metrangolo V, Engelholm L H. Antibody–Drug Conjugates: The Dynamic Evolution from Conventional to Next-Generation Constructs. Cancers 2024, 16, 447. https://doi.org/10.3390/cancers16020447
- Walsh S J, Bargh J D, Dannheim F M, et al. Site-selective modification strategies in antibody–drug conjugates. Chemical Society Reviews 2021, 50(2): 1305-1353. https://doi.org/10.1039/D0CS00310G. Distributed under Open Access license CC BY 3.0, without modification.
- Song C H, Jeong M, In H, et al. Trends in the development of antibody-drug conjugates for cancer therapy. Antibodies 2023, 12, 72. https://doi.org/10.3390/antib12040072 Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only. NOT FOR CLINICAL USE.

Online Inquiry
Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
