- Home
- Resources
- Knowledge Center
- Literatures
- Development & Validation of ELISA for the Quantification of MMAE-Conjugated ADCs & Total Antibodies
Development and Validation of ELISA for the Quantification of MMAE-Conjugated ADCs and Total Antibodies
Overview
Despite the widespread use of the antimitotic agent monomethyl auristatin E (MMAE) as an ADC payload for multiple ADCs, simultaneous quantitative detection of MMAE-conjugated ADCs and total antibodies remains challenging. Therefore, the development of simple and high-throughput quantitative analysis assays, such as enzyme-linked immunosorbent assays (ELISA), is crucial for monitoring their stability and pharmacokinetic (PK) characteristics.
MMAE, a tubulin inhibitor, serves as a toxin payload in antibody-drug conjugates. Creative Biolabs boasts extensive experience in the field of ADC development and offers customized ADCs development using microtubule toxins as payloads, including auristatins, epothilone, maytansinoids, taxoids, tubulysins, and vinorelbine.
In this study, a specific anti-MMAE monoclonal antibody (mAb) with a high binding affinity for MMAE was generated. Utilizing this antibody, sensitive and high-throughput ELISA assays were developed, validated, and applied to measure the concentrations of MMAE-conjugated ADCs and total antibodies (tAb, antibodies in ADC plus unconjugated antibodies) in cynomolgus monkey sera. As a typical successful case, these assays were applied to in vitro plasma stability and pharmacokinetic (PK) studies of TROP2-ADC, an MMAE-conjugated ADC targeting trophoblast cell surface antigen 2 (TROP-2).
Preparation and Characterization of Anti-MMAE mAb
Bovine serum albumin (BSA) was conjugated to the Val-Cit-PAB-MMAE linker-drug to generate the BSA-Val-Cit-MMAE immunogen (see Fig. 1). BALB/c mice were subcutaneously injected with 50 μg of BSA-Val-Cit-MMAE immunogen emulsified in complete Freund's adjuvant (CFA). Spleen cells from immunized mice were fused with mouse SP2/0 myeloma cells at a 5:1 ratio. Hybridoma supernatants were screened for binding to the MMAE-conjugated sacituzumab antibody and BSA-Val-Cit-MMAE. Hybridomas that tested positive for MMAE conjugates were expanded and then assessed for binding to the BSA-Val-Cit-MMAE immunogen using ELISA. The cross-binding positive cell clones were utilized to obtain the heavy chain and light chain variable region sequences of the candidate anti-MMAE mAb through PCR. After aligning with the sequence of the patented antibody, the DNA fragment was cloned into pCAT1.0 and pCAT2.0, and transiently transfected into HEK293F cells using polyethyleneimine. Supernatants were collected after 6 days of culture and purified via protein A affinity chromatography.
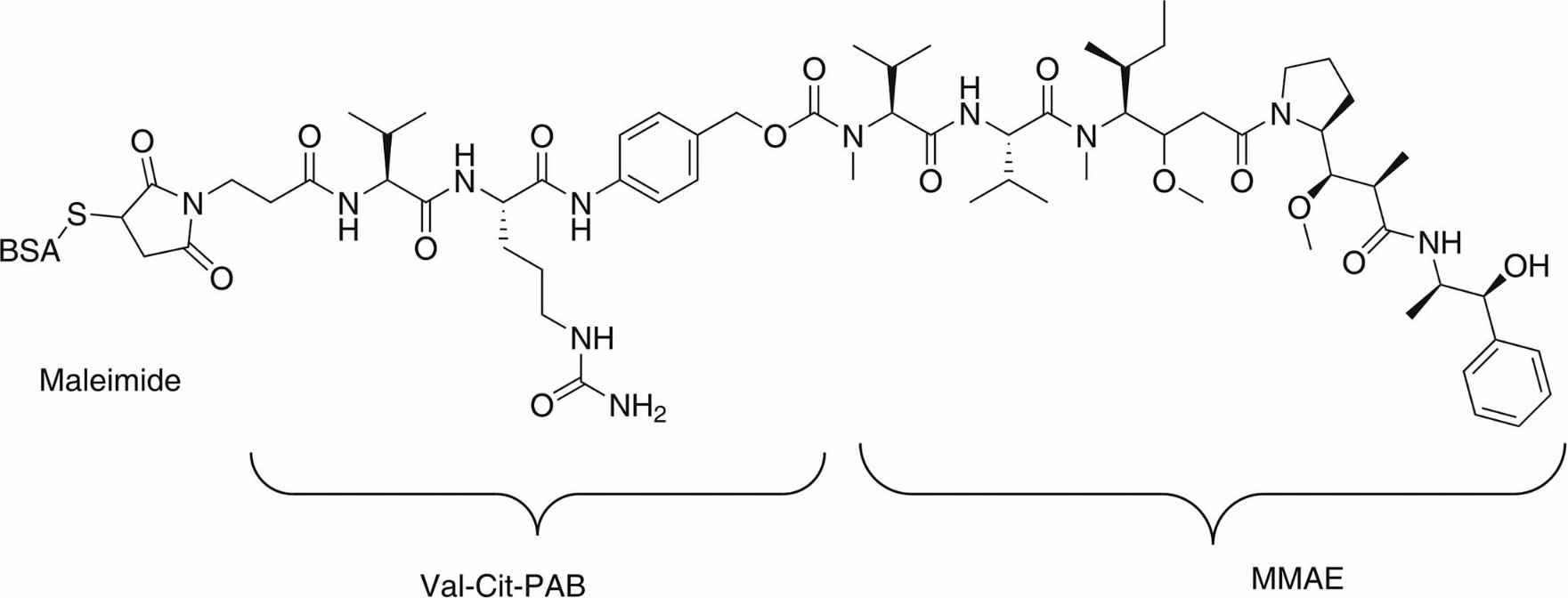 Fig. 1 Chemical structure of monomethyl auristatin E-bovine serum albumin (MMAE-BSA) immunogen.1
Fig. 1 Chemical structure of monomethyl auristatin E-bovine serum albumin (MMAE-BSA) immunogen.1
SDS-PAGE, SEC, and ELISA were employed to investigate the quality, purity, and binding affinity of the anti-MMAE mAb, respectively. Experimental data demonstrated that the light and heavy chains were approximately 25 and 50 kDa, respectively (see Fig. 2A), and the purity of the mAb was nearly 100% (see Fig. 2B). The mAb exhibited a high affinity for TROP2-ADC (see Fig. 2C), with an EC50 of 0.120 μg/mL.
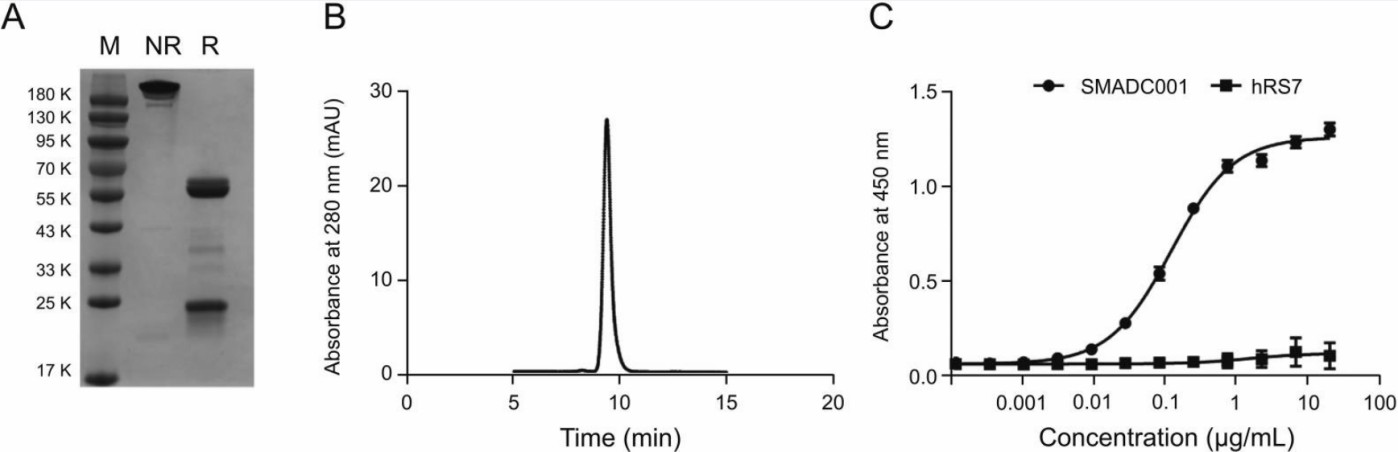 Fig. 2 Biochemical characterization of purified anti-MMAE monoclonal antibody (mAb).1
Fig. 2 Biochemical characterization of purified anti-MMAE monoclonal antibody (mAb).1
Creative Biolabs possesses extensive experience in ADC antibody screening and characterization. We have the capability to assist you in screening for antibodies with high antigen specificity and binding affinity using our well-established phage display platform, as well as offering antibody humanization services.
Biochemical and Pharmacological Characterization of TROP2-ADC
A direct ELISA was employed to evaluate the binding affinity of TROP2-ADC to the hTROP-2-ECD-His protein. The conjugation reaction yielded a product with a purity of 98.3%, as confirmed by SEC analysis (see Fig. 3B). The average DAR value was 4.3. TROP2-ADC exhibited high affinity binding to the hTROP-2-ECD-His protein, with an EC50 value of 0.0112 μg/mL, as determined by direct ELISA (see Fig. 3C). It showed potent antitumor activity against TROP-2-expressing BXPC-3 cells, with an IC50 value of 0.128 nM. These results suggest that TROP2-ADC holds potential as an effective antitumor agent (see Fig. 3D).
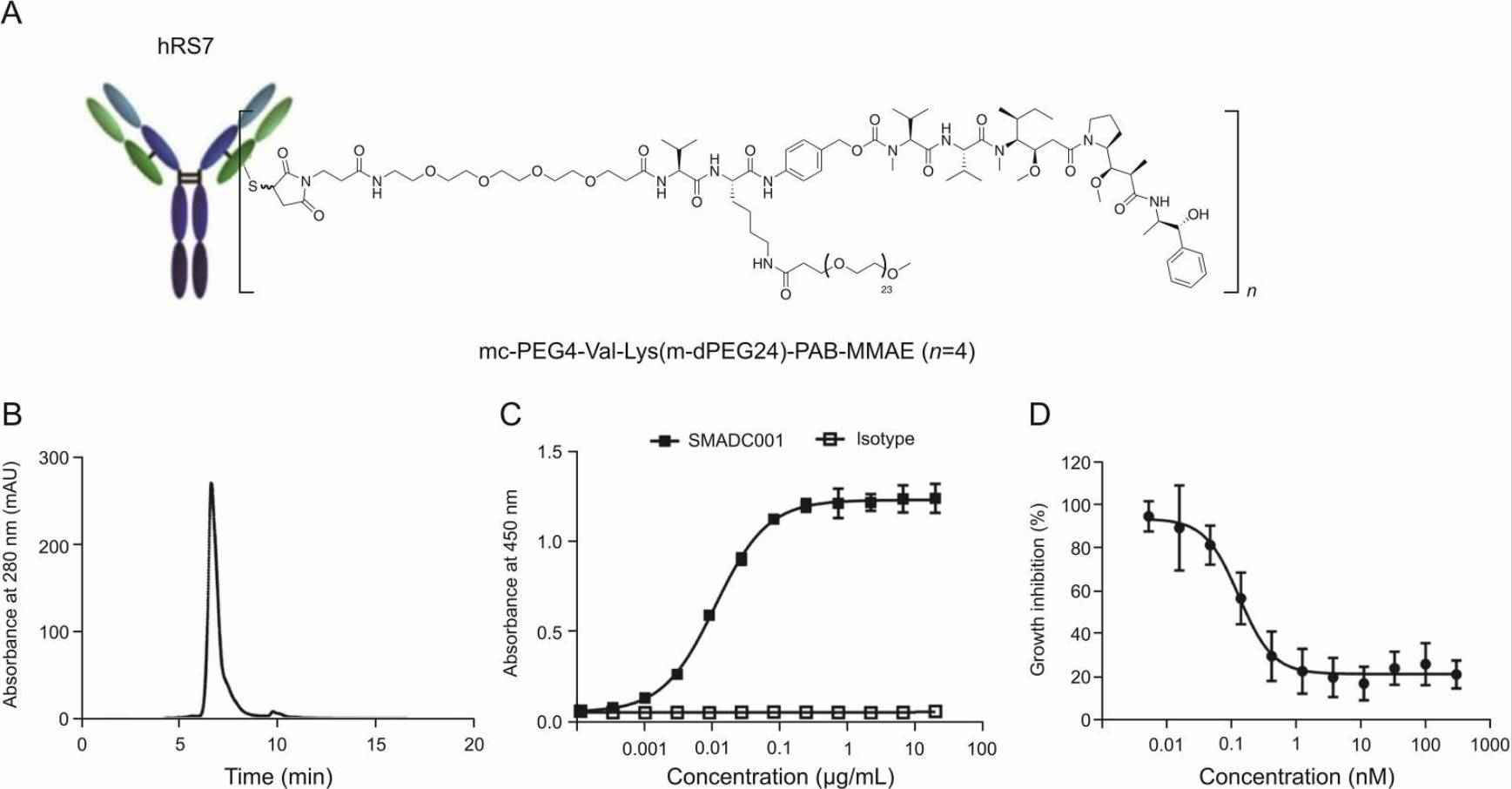 Fig. 3 Structure and characterization of TROP2-ADC.1
Fig. 3 Structure and characterization of TROP2-ADC.1
Sandwich ELISA Procedure
Three different sandwich ELISA procedures were established to measure TROP2-ADC ADC, TROP2-ADC tAb, and MMAE. Methodological verification was performed, including intra- and inter-assay precision and accuracy, matrix effect and selectivity, as well as plasma stability.
For the calibration standards of ADC and tAb, these ELISA methods revealed a strong correlation between serum concentrations and the OD450 values, with an R2 of 1.000. The dynamic range was 0.3-35.0 ng/mL and 0.2-22.0 ng/mL, respectively. Intra- and inter-assay accuracy bias% ranged from -12.2% to 5.2%, while precision ranged from -12.4% to -1.4%The relative standard deviation (RSD) was less than 6.6% and 8.7%, respectively. The total error remained below 20.4%. The results from the development and validation steps of these two assays indicate their suitability for quantifying MMAE-conjugated ADCs and for use in PK studies.
PK Studies of TROP2-ADC in Cynomolgus Monkeys
The PK profiles of TROP2-ADC were assessed in cynomolgus monkeys following a single intravenous infusion. Four cynomolgus monkeys (weighing 2.8-6 kg) were divided into two groups: the vehicle group (injected with TROP2-ADC buffer) and the TROP2-ADC group (injected with 3 mg/kg TROP2-ADC via intravenous infusion (hindlimb vein, 30 min/dose/monkey).
The PK curves for TROP2-ADC, tAb, and MMAE in cynomolgus monkeys treated with 3 mg/kg TROP2-ADC are shown in Fig. 4. Serum concentrations of TROP2-ADC, tAb, and free MMAE were measured as the study set procedure. The serum concentration of TROP2-ADC closely mirrored that of tAb over the entire time course, consistent with the results of free MMAE measurements. This suggests that the developed ELISAs are sufficiently capable of quantifying MMAE-conjugated ADCs and tAbs for pharmacokinetic studies in cynomolgus monkeys.
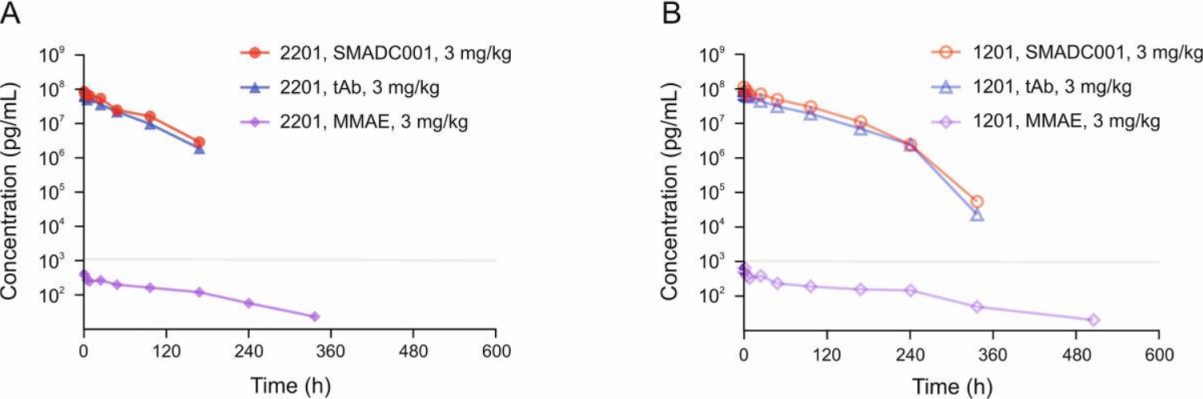 Fig. 4 Pharmacokinetic profiles of TROP2-ADC, tAb, and free MMAE in cynomolgus monkeys following a single intravenous infusion (3 mg/kg) of TROP2-ADC.1
Fig. 4 Pharmacokinetic profiles of TROP2-ADC, tAb, and free MMAE in cynomolgus monkeys following a single intravenous infusion (3 mg/kg) of TROP2-ADC.1
Creative Biolabs provides one-stop ADC development services tailored to your specific needs, serving as your one-stop solution for in vitro and in vivo ADC analysis.
Reference
- Pei M, Liu T, Ouyang L, et al. Enzyme-linked immunosorbent assays for quantification of MMAE-conjugated ADCs and total antibodies in cynomolgus monkey sera. J Pharm Anal. 2022, 12(4):645-652.
For Research Use Only. NOT FOR CLINICAL USE.

Online Inquiry
Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
