- Home
- Resources
- Knowledge Center
- Reviews
- Tumor Target Screening in ADC Drug Development
Tumor Target Screening in ADC Drug Development
Currently, there are different antibody-drug conjugates (ADCs) in the world, and they are transforming disease therapy. ADCs are a revolutionary category of cancer medicines today, where the selectivity of monoclonal antibodies is being combined with the potency of cytotoxic payloads. At the heart of their success lies the careful selection of tumor-specific targets, which determines efficacy, safety, and therapeutic promise. Target selection is the most crucial issue of ADC development.
According to comprehensive database analyses and recent reports, over 80% of ADC drugs currently under investigation are being explored for therapeutic applications in solid tumor therapeutics. These agents target similar or distinct tumor-associated antigens, with some demonstrating promising clinical trial outcomes while others exhibit suboptimal experimental results. Figure 1 delineates ADC targets that have encountered clinical attrition at different trial phases. This empirical evidence provides critical guidance for rational target selection in subsequent investigational efforts.
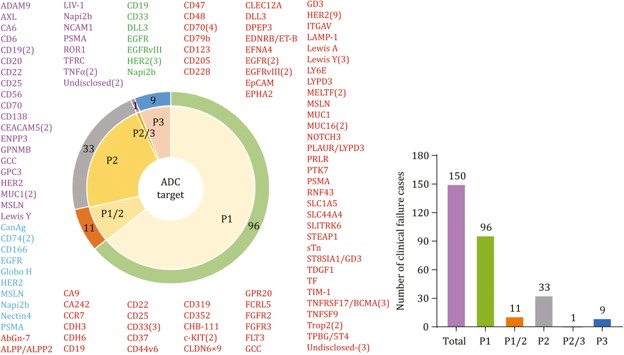 Fig 1. Antigen targets of clinically tested ADCs.1,5
Fig 1. Antigen targets of clinically tested ADCs.1,5
Target Selection for ADCs
The ideal ADC target must express highly tumor-specifically and be efficiently internalized to deliver maximum payload with minimum off-target toxicity. This balance is seen in tumor antigens like HER2 and TROP2. Overexpressed in 15–30% of breast and gastric cancers, HER2 supports clathrin-mediated endocytosis with swift internalization, ensuring efficient toxin release. Similarly, the constitutive internalization of TROP2 in tumors enhances the potency of ADCs, as evidenced by sacituzumab govitecan's activity against triple-negative breast cancer. Low antigen density, however, is not invariably excluding efficacy. There are drugs that are demonstrated to be active even in HER2-low breast cancer, which challenges the concept of an expression threshold being absolute. This strongly supports empirical determination of target expression cut-offs that will be tumor type and ADC design specific.
Antigens demonstrating substantial overexpression in malignant neoplasms yet minimal expression in normal tissues are preferentially considered as ADC targets. Identifying targets that fully satisfy these stringent criteria remains challenging, necessitating strategic incorporation of two or more complementary targets in ADC formulations. The development of multi-targeted ADCs has emerged as a pivotal strategy for expanding therapeutic windows. This approach involves systematic prioritization of molecular targets exhibiting significant overexpression in specific malignancies through comparative analyses of mRNA transcript abundance and protein expression disparities between tumor and normal tissues. During target validation, concurrent co-expression correlation studies are implemented to elucidate target interaction networks. Strategic combination of either synergistically co-expressed targets or distinct epitopes on the same antigenic molecule has demonstrated enhanced ADC specificity and improved intracellular trafficking efficiency. To mitigate off-target risks inherent in multi-target ADCs, rigorous screening mandates comprehensive evaluation of candidate targets' distribution profiles across normal tissues, explicitly excluding antigen combinations with co-localized expression in shared healthy organs. Advanced functional genomics strategies further enable identification of tumor-specific antigens critical for neoplastic cell viability yet non-essential in normal cells. Synergistic integration of such functionally validated targets permits construction of dual/multi-targeting ADC systems. This innovative paradigm effectively circumvents off-target toxicities associated with conventional mono-targeted therapies.
Tumor-associated Antigen Expression of Approved ADCs
Tumor-associated antigens (TAAs) exhibit tumor-specific overexpression patterns in malignant cells. As illustrated in Figure 2, this study constructed a pan-dimensional expression profile of clinically approved ADC-related TAAs through systematic analysis of transcriptomic data. The investigation revealed that hematologic malignancy-targeting biomarkers (CD33, CD19, CD22) demonstrate marked selective enrichment in their corresponding tumor types while maintaining physiologically restricted expression in other tissues. In contrast, solid tumor-associated targets, including FR-α, Trop-2, Nectin-4, and TF, demonstrate broader basal expression levels across normal tissues, highlighting substantial heterogeneity in TAA distribution patterns between distinct tumor types.
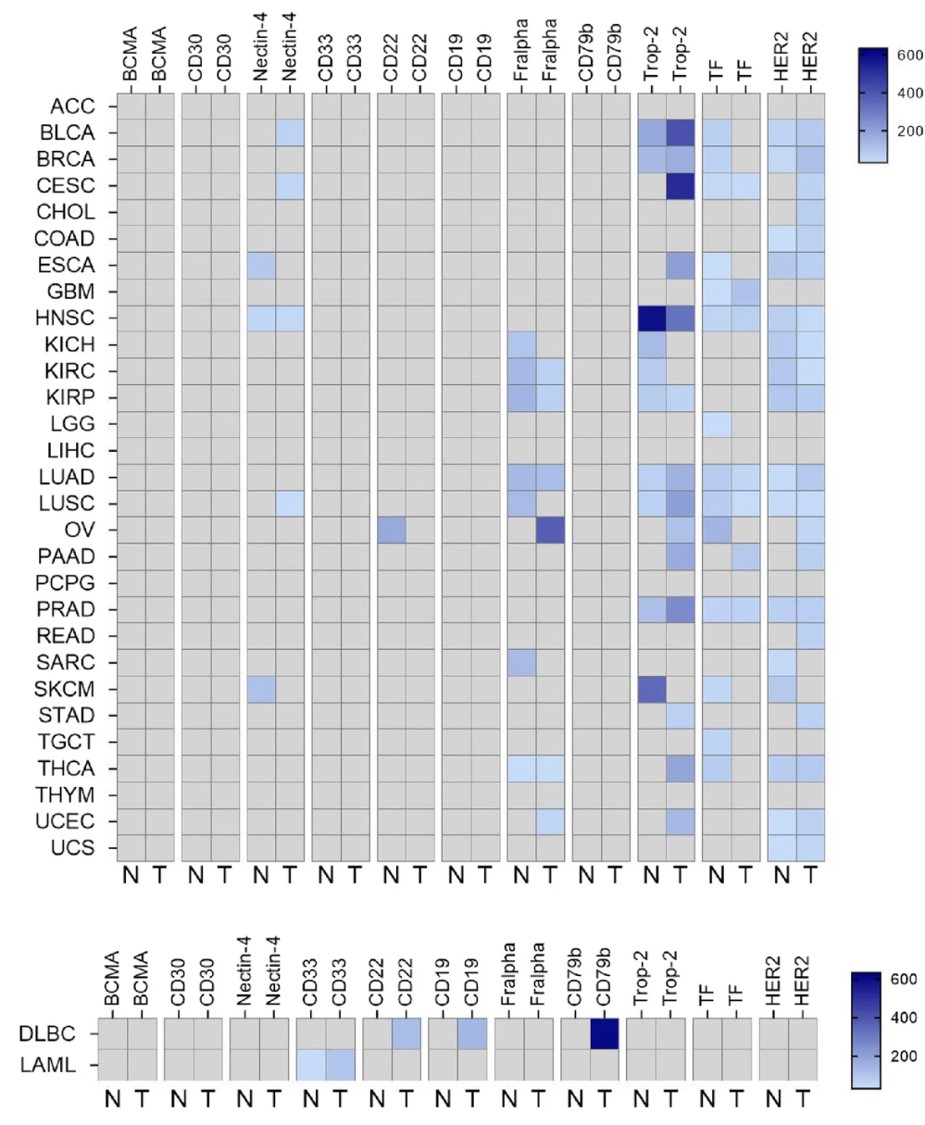 Fig 2. Tumor-associated antigen expression of approved ADCs across different indications.2,5
Fig 2. Tumor-associated antigen expression of approved ADCs across different indications.2,5
Key ADC Targets
HER2
The transmembrane tyrosine kinase receptor HER2 (ERBB2) persists as a strategic priority in ADC development, driven by its pan-cancer overexpression profile and unique structural determinants enabling efficient receptor-mediated endocytosis. Mechanistically, HER2's ligand-independent dimerization propensity and rapid lysosomal trafficking kinetics create optimal pharmacodynamic conditions for ADC payload delivery, particularly in breast carcinomas exhibiting ERBB2 amplification.
HER2 oncoprotein is a surfacing recurring genomic abnormality across several malignancies, including salivary gland neoplasms, colorectal and biliary tract cancers, and also urothelial carcinomas, and even non-small cell lung malignancies. Cell surface receptor tyrosine kinase has become a predominant therapeutic axis both as a successful target for marketing-approved ADCs and as the central area under investigation pipelines currently. While HER2-targeted biologics are associated with potent survival advantages in breast and stomach cancer, their clinical efficacy significantly wanes in the case of other HER2-amplified solid tumors. Such therapeutic discord necessitates new targeting strategies for enhancing tumor-specific binding affinity. Novel clinical experience in molecularly defined subgroups of colorectal cancer illustrates enhanced therapeutic responses with agents targeting HER2, indicating potential reclassification of treatment protocols in precision oncology platforms.
CLDN18.2
Claudin 18.2 (CLDN18.2), a gastric-specific tight junction protein, offers a niche for ADC development. CLDN18 modulates paracellular permeability, polarity, and signaling. CLDN18 deficiency has been associated with atrophic gastritis, spasmolytic polypeptide-expressing metaplasia (SPEM), and asthma. Not expressed in other healthy tissues, gastric mucosa-specific isoform CLDN18.2 expression manifests in primary gastric cancers. CLDN18.2 at the surface of epithelial tumor cells is a target in antibody and CAR-therapies currently in development. However, challenges include antigen heterogeneity and the need for precise patient stratification to avoid off-target effects in normal gastric mucosa.
TROP2
Trophoblast cell surface antigen 2 (Trop2), a transmembrane glycoprotein gene product of TACSTD2, plays a pivotal role in embryonic morphogenesis through the modulation of calcium signals. Constitutive expression of Trop2 has been found by immunohistochemical profiling in a wide range of physiological systems, including but not limited to: stratified squamous epithelium of the cervix, integumentary keratinocytes, esophageal mucosa, and tonsillar crypt architecture. This glycoprotein is a molecular footprint of glandular epithelia since it is constitutively expressed in cuboidal/columnar epithelia that line glandular and ductal types of mammary, prostatic, and pancreatic tissues. Pulmonary pneumocytes and renal medullary epithelia express detectable basal Trop2 expression, and high expression profiles are observed in mammary ductal epithelial cells. Oncologically, Trop2 overexpression is a pathognomonic feature among several malignancies. Therefore, Trop2 has emerged as a very promising and strategic candidate for the development of ADCs towards therapeutic purposes.
CEACAM5
CEACAM5 (CD66e/CEA), a GPI-anchored glycoconjugate member of the immunoglobulin superfamily's CEACAM cluster, constitutes a paradigm of oncodevelopmental antigens with dual diagnostic and pathophysiological significance. Its clinical utility as a circulating tumor marker is particularly established in metastatic colorectal carcinoma management, demonstrating >90% specificity in stage III-IV CRC cohorts. While exhibiting tight spatiotemporal regulation in non-pathological states, comprehensive tissue atlasing reveals CEACAM5's polarized membrane topology varies significantly across epithelial subtypes: apicolateral localization in intestinal absorptive enterocytes and mucin-producing goblet cells, correlating with Wnt/β-catenin signaling gradients; basolateral patterning in glandular epithelia of salivary/submandibular ducts; diffuse membranous expression in stratified squamous epithelia of upper aerodigestive tract; and constitutive low-level expression in epidermal basal layer and renal glomeruli, potentially mediating epithelial-mesenchymal crosstalk through CEACAM5-NCAM interactions.
The clinical utility of CEACAM5 primarily resides in gastrointestinal oncology, where its overexpression in colorectal adenocarcinoma serves dual diagnostic and prognostic biomarker functions. The differential expression profile between malignant and normal tissues has positioned CEACAM5 as a strategic candidate for targeted therapeutic development, particularly in ADC engineering and T-cell redirecting bispecific antibody platforms. Current investigational approaches focus on leveraging its 10-50 fold overexpression gradients observed in epithelial carcinomas compared to adjacent normal epithelia, while implementing stringent exclusion criteria for therapeutic constructs targeting organs exhibiting physiological CEACAM5 expression.
B7-H3
B7-H3 (CD276), an immune checkpoint molecule, is overexpressed in NSCLC, nasopharyngeal carcinoma (NPC), and small-cell lung cancer (SCLC). Its success highlights B7-H3's dual role in tumor killing and immune modulation, positioning it as a frontier target for combinatorial therapies.
Service you may interested in
Trends in ADC Drug Development
Collectively, second-generation site-specific conjugation technologies enable the installation of defined bioorthogonal moieties onto antibodies to enable directed payload attachment at engineered sites. Installation of such functional motifs does entail some level of structural perturbation of the antibody scaffold that may have unforeseen effects on its conformational stability, folding kinetics, or antigen-binding specificity, posing a serious challenge to downstream manufacturing process, including optimization of batch-to-batch consistency, reduction of aggregation, and evaluation of functionality of the engineered constructs.
Target selection remains the linchpin of ADC success, requiring meticulous evaluation of tumor specificity, internalization dynamics, and safety profiles. While established targets like HER2 and TROP2 dominate current pipelines, innovation in dual-targeting, novel antigens, and adaptive designs will drive the next wave of ADC therapeutics. As the field evolves, integrating dynamic biomarker monitoring and personalized dosing strategies will unlock the full potential of these "magic bullets" in precision oncology.
Unleash ADC Research Potential – Consult Our Experts Now
Are you eager to explore the identification of potential target sites for ADC candidates? Unlock the full potential of your research with the expertise of Creative Biolabs! Our team of seasoned professionals is ready to guide you through every step of the process, offering in-depth insights and tailored solutions. Don't miss out on this opportunity to gain a competitive edge in the field of antibody-drug conjugates. Contact our experts today and take the first step towards groundbreaking discoveries!
References
- Liu, H.; Zeng, H.; Qin, X.; et al. The Icarian flight of antibody-drug conjugates: target selection amidst complexity and tackling adverse impacts. Protein & Cell, 2025, pwaf002. https://doi.org/10.1093/procel/pwaf002
- López de Sá, A.; Díaz-Tejeiro, C.; Poyatos-Racionero, E.; et al. Considerations for the design of antibody drug conjugates (ADCs) for clinical development: lessons learned. J Hematol Oncol 2023, 16, 118. https://doi.org/10.1186/s13045-023-01519-0
- Criscitiello, C.; Morganti, S.; Curigliano, G. Antibody–drug conjugates in solid tumors: a look into novel targets. J Hematol Oncol, 2021 14, 20. https://doi.org/10.1186/s13045-021-01035-z
- Esapa, B.; Jiang, J.; Cheung, A.; et al. Target antigen attributes and their contributions to clinically approved antibody-drug conjugates (ADCs) in haematopoietic and solid cancers. Cancers, 2023, 15(6): 1845. https://doi.org/10.3390/cancers15061845
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only. NOT FOR CLINICAL USE.

Online Inquiry
Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
