- Home
- ADC Development
- ADC In Vitro Analysis
- ADC Biochemical Analysis
- ADC Stability Analysis
- ADC Plasma Stability Analysis
ADC Plasma Stability Analysis Service
Antibody drug conjugates (ADCs) are a unique class of therapeutic anti-cancer agents in which cytotoxic drugs are covalently conjugated to a monoclonal antibody through small chemical linkers. The idea behind an ADC is the targeted delivery of cytotoxins to tumors using blood circulation as the transportation media. Thus, the plasma stability of an ADC is of crucial importance since it dictates the off-target drug release and directly affect its in vivo efficacy and safety. Creative biolabs fully understands the role of plasma stability in the ADC development and we have adopted an advanced analytical system to enable its accurate evaluation.
The plasma stability of an ADC is affected by several factors, including the linker chemistry, conjugated sites, and drug to antibody ratio (DAR) distribution.
- Linker chemistry
Linker stability is crucial to avoid premature payload releases before the ADCs reach their desired targets. Therefore, linkers should originally be designed for maximum blood stability. For ADC developments, non-cleavable linkers usually exert good plasma stability while for cleavable linkers (peptide linkers, β-glucuronide linkers, pH-sensitive linkers, and Glutathione-sensitivity linkers), their plasma stability need further evaluation before in vivo efficacy analyses. Lessons learned from the early ADC developments using hydrazones or disulfide linkers that showed significant off-target drug release and led to severe cytotoxicity. The replacement of such linkers with an enzyme cleavable dipeptide Val-Cit linker greatly improved the ADC plasma stability and reduced the side-effects associated with premature off-target payload releases.
- Conjugation sites
Although the plasma stability and potentially the efficacy of an ADC can be improved by selecting the appropriate antibody and payload and by optimizing the linker chemistry, the chemical and structural dynamics of the conjugation sites can also have an influence on the ADC performance by regulating the stability of the antibody-linker interface. In terms of maleimide-containing drug-linker complexes, early studies have shown that conjugation sites with high solvent accessibility rapidly lost the conjugated thiol-reactive linkers and payloads into plasma due to the linker-maleide exchanges from the ADC to serum albumin, free cysteines, or with glutathione. In contrast, partially solvent accessible sites within a positively charged environment facilitate the hydrolysis of the succinimide ring in the linker, thereby stabilizing the local structure and preventing the exchange reaction.
- Drug to antibody ratio distribution
Drug to antibody ratio (DAR) is also a key element in plasma stability. The conjugate species with higher DARs exert rapid circulation clearance than the lower DAR species.
Here at Creative Biolabs, we assay both in vitro and in vivo ADC plasma stabilities:
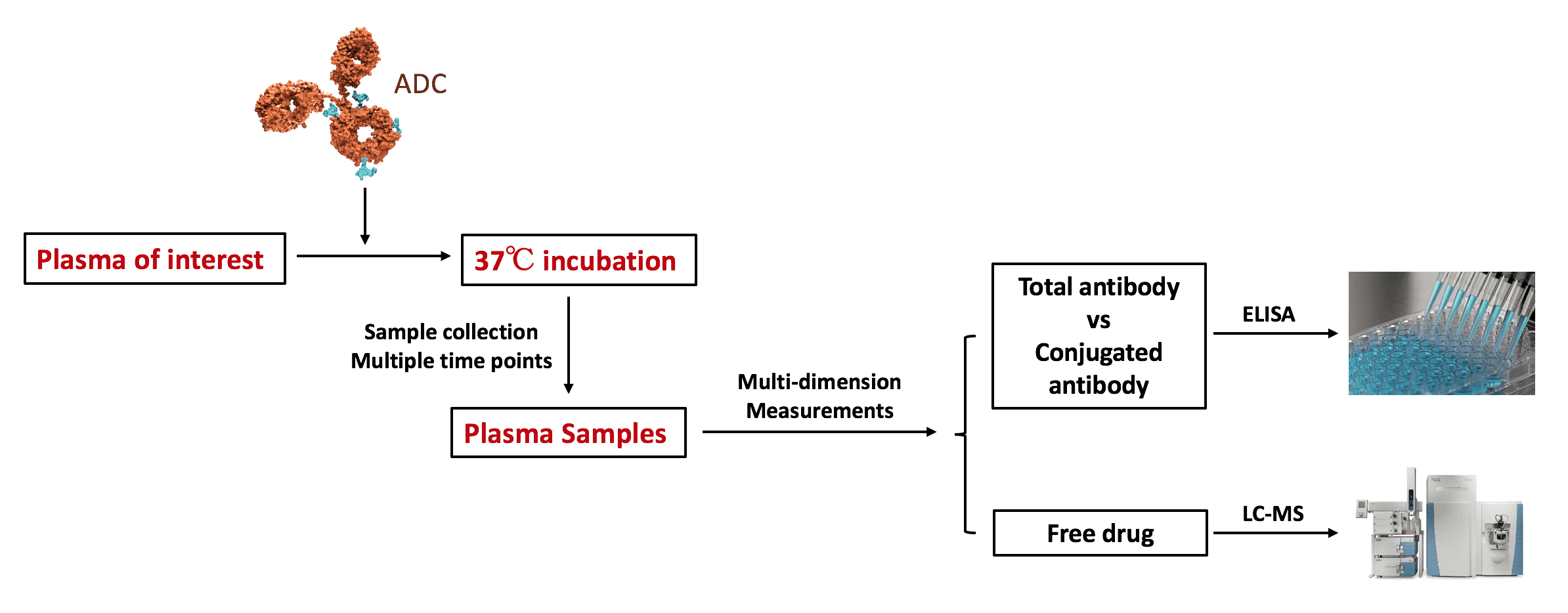 A flow chart representing a general procedure for plasma stability analysis.
A flow chart representing a general procedure for plasma stability analysis.
- In vitro plasma stability analysis
To assess the in vitro plasma stability of an ADC, the ADC sample is incubated in plasma from different species at 37oC for a period of time. Plasma samples are drawn at different time points and the amount of total antibody and conjugated antibody are measured using ELISA to calculate the degree of drug loss. In the meantime, the free drug or drug-linker set in the plasma samples are quantified using LC-MS to confirm the results from ELISA analysis.
- In vivo plasma stability analysis
The In vivo plasma stability analysis is similar to that of the in vitro assay, with the difference being the ADC is injected and incubated within an appropriate model animal. Blood samples are drawn at certain time points and the drug release is monitored using similar approaches as that in the in vitro assay.
As a recognized industrial leader in antibody engineering, bio-conjugation, and ADC development, Creative Biolabs is committed to provide our clients with the most comprehensive analysis services for ADC characterization and we also offer various other services to be your one-stop service station for ADC development. Please contact us for more information and a detailed quote.
For Research Use Only. NOT FOR CLINICAL USE.

Online Inquiry
Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
