- Home
- Resources
- Knowledge Center
- Literatures
- Clinical Toxicity of ADC: A Meta-Analysis of Payloads
Clinical Toxicity of ADC: A Meta-Analysis of Payloads
The Payload & Dose-Limiting Toxicitie (DLT)
Despite new drug research and development interest in ADCs, designing a successful ADC with an improved therapeutic index has been quite challenging due to dose-limiting toxicities (DLTs). Considering that the recommended dose of an ADC is typically derived from the maximum tolerated dose (MTD) determined by DLTs that are primarily associated with the payload, it is necessary to leverage the clinical experience from one ADC to inform the likelihood of observing those same DLTs in a novel ADC with the same payload or linker-payload.
Creative Biolabs provides one-stop ADC development services with complete and scientific solutions according to your requirements.
In this article, the model-based meta-analysis was described using statistical methods to combine and quantify the outcomes of a series of clinical trials in a single pooled analysis. On this basis, a dose-response model for severe-grade toxicity incidence was established as a function of payload, dose/regimen, and cancer type (solid tumor vs. hematologic cancer) by summarizing the key clinical safety data published for ADCs by payload class.
Method
The authors identify and extract data from clinical ADC studies by conducting a literature search. Endpoints of interest (listed below) are common adverse events (AEs) that, when severe, are often considered DLTs to determine the MTD of ADCs in clinical development.
- Anemia
- Neutropenia
- Thrombocytopenia
- Leukopenia
- Hepatic toxicity (including liver enzyme elevation)
- Peripheral neuropathy
- Ocular toxicity
In addition, dosage, dosing regimen/frequency, patient population and demographic data (including biomarker status, prior treatment, first-line vs. relapsed/refractory treatment, age, sex, race, etc.), and other ADC data (if any) were collected to study.
Data from 70 studies were included in this analysis, and of the 70 studies, 50 reported grade 3 and/or 4 toxicity information for at least 1 safety endpoint of interest in at least 1 dose group. 43 of 50 studies (86%) reported incidence in the Ball patient^ group, and four payload classes, including DM1, DM4, MMAE, and MMAF, were present in the dataset from 43 studies.
Data Analysis
The authors analyzed to compare the incidence of G3/4 for each toxicity endpoint across ADC payload categories. Use exploratory plots (qualitative assessment), descriptive summaries (quantitative assessment), and modeling (quantitative assessment) to explore G3/4 toxicity and assess the impact of covariates, such as cancer type and dose/regimen.
Forest plots were used to display G3/4 toxicity incidence by payload category and cancer type (solid tumors vs. hematological malignancies vs. both types combined) and included toxicity point estimates and 80% confidence intervals using the Agresti-Coull method.
All levels of toxicity were also quantitatively assessed by payload category. Statistical modeling was performed using mixed-effects logistic meta-regression with the payload category as the main structural variable.
Results
Grouped by payload and using anemia toxicity as a representative case analysis, forest plots (shown in Fig. 1) show the percentage of anemic G3/4 toxicity reported for each study treatment group (ID) and sample size (N). Other AEs were analyzed similarly.
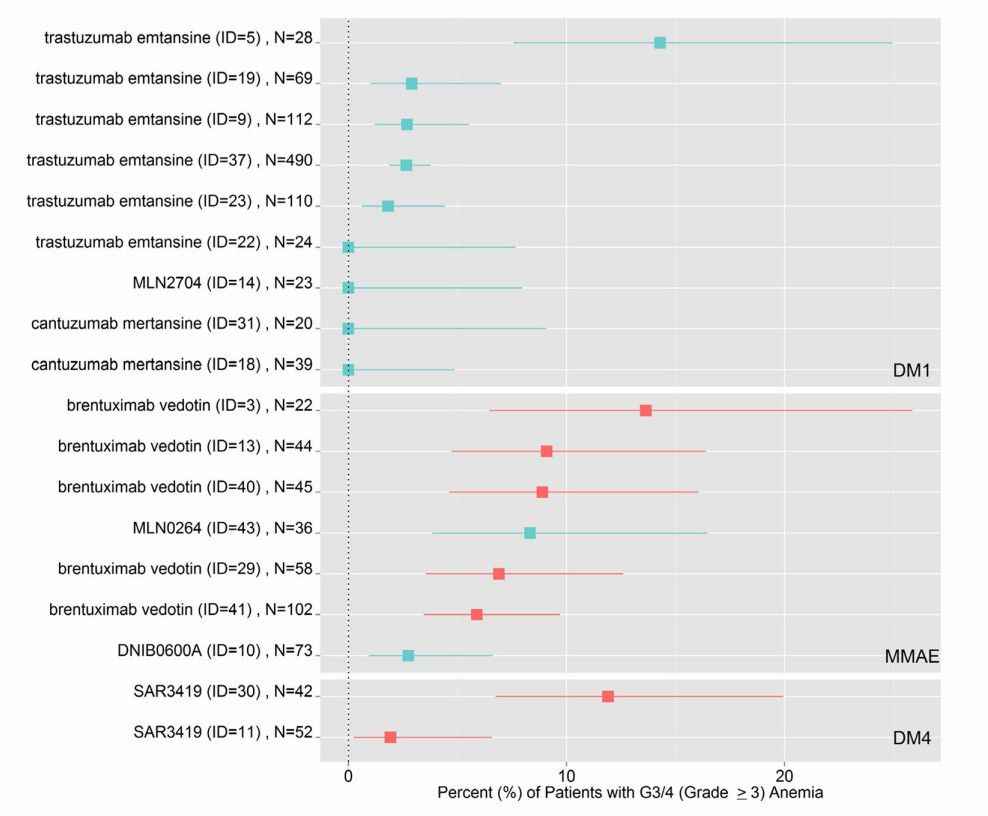 Fig. 1. The percent of G3/4 toxicity for anemia reported for individual study treatment arms (ID) and sample size (N). (Masters JC, et al., 2018)
Fig. 1. The percent of G3/4 toxicity for anemia reported for individual study treatment arms (ID) and sample size (N). (Masters JC, et al., 2018)
- Results show that some payloads are associated with specific critical AEs at severity level (G3/4). The MMAE ADC consistently reported severe anemia (Fig.1), the MMAE reported neutropenia, and DM1 reported thrombocytopenia. Regarding serious non-hematological AEs, hepatotoxicity (primarily manifesting as increased AST and/or ALT) was consistently reported with DM1 ADCs, while peripheral neuropathy was reported with MMAEs.
- However, for certain AE endpoints, safety trends for MMAE, DM1, and DM4 ADCs appeared to differ between solid and hematologic cancer indications.
- On this basis, logistic regression estimates (±90% confidence limits) of the incidence of G3/4 reactions according to toxicity and payload categories were performed. The payload category is the main covariate modeled. Results showed that ADCs with MMAE or DM4 as payloads consistently reported grade 3 or higher hematological toxicities (≥5% for each toxicity), whereas in the remaining payload categories, these types of toxicities are not as frequent.
- The authors further analyzed the qualitative representation of G3/4 toxicity observed in cancer types, showing that the toxicity profile within a given payload may differ depending on the population of certain payloads.
Overall, this analytical work demonstrates the opportunity to improve the status quo of non-selective, off-target toxicity through enhanced ADC chemical design, thereby supporting the determination of optimal ADC doses and dosing regimens for target patient populations.
Creative Biolabs is a company focused on ADC development. We have comprehensive technical capabilities and can manipulate multiple payloads to enhance ADC chemical design. At the same time, we also provide comprehensive and highly customizable safety assessment services, including single-dose toxicity, repeated dose toxicity, maximum dose toxicity, etc., to meet customers' needs for safety data.
Reference
- Masters JC, Nickens DJ, Xuan D,et al. Clinical toxicity of antibody drug conjugates: a meta-analysis of payloads. Invest New Drugs. 2018,36(1):121-135.
For Research Use Only. NOT FOR CLINICAL USE.

Online Inquiry
Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
