Antigen Microarray Detection Service for SARS-CoV-2 Coronavirus
As a pioneer in NAA profiling, Creative Biolabs is proud to introduce our services of antigen microarray detection for SARS-CoV-2 coronavirus. With up-to-date technologies and a team of highly experienced experts, a novel and mature NAA profiling platform has been established here to provide autoantigen microarray detection services, which supports performing functional and analytical assays for our global customers, exclusively assisting you in your research project.
Background
Introduction of SARS-CoV-2 Coronavirus
The genetic material of the SARS-CoV-2 is an RNA including six open reading frames (ORFs) responsible for the production of the nucleocapsid (N), spike (S), membrane (M), and a small envelope (E) structural protein subunits; the rest of the genes produce 16 functional proteins such as RNA dependent RNA polymerase (RdRP) and helicase. N protein attaches to the coronavirus genetic RNA and forms nucleocapsid. Spike glycoproteins (S1 and S2 subdomains) are surface densely glycosylated proteins specifying the type of the infected host and are involved in various tendencies to different tissues.
Angiotensin-converting enzyme 2 (ACE2), expressed in human endothelial cells in the lung, intestine, heart, and kidney, is a surface protein and a functional receptor for coronavirus. This protein acts as a direct binding site for virus S protein, the receptor-binding domain (RBD) of the S1 subunit of S protein directly contacts the ACE2 receptor with a high affinity and has crucial roles in the attachment and fusion of the virus through the ACE2-containing host cells. M protein has the main role in forming new virus particles and could insert some proteins from the host into the viral envelope. The E protein is the smallest structural protein having roles in coronavirus assembly and pathogenesis. The arrangement of these four proteins is different among coronaviruses, but their presence is critical for the infectious characteristics of SARS-CoV-2. By knowing these facts, identifying, and considering these potential risk factors could help the physicians for a better prognosis and diagnosis.
Antigen Microarray Detection for SARS-CoV-2 Coronavirus
Microarray detection is a high throughput technique with efficient performance in pathogen detection and quick results. Production of cDNA labeled with specified probes via reverse transcription from the coronavirus RNA is a pre-requisite for this method. These labeled cDNAs are then loaded into wells and are hybridized with fixed oligonucleotides in the solid phase on the microarray. This step is then followed by sequential steps of washing to omit the free DNAs. The detection of RNA from CoV is followed by using specified probes. The rapidity, specificity, and accuracy of microarray-based detection make it a superior choice for CoV detection. This test requires a high cost therefore, low-density non-fluorescent oligonucleotides are developed which can minimize the expense.
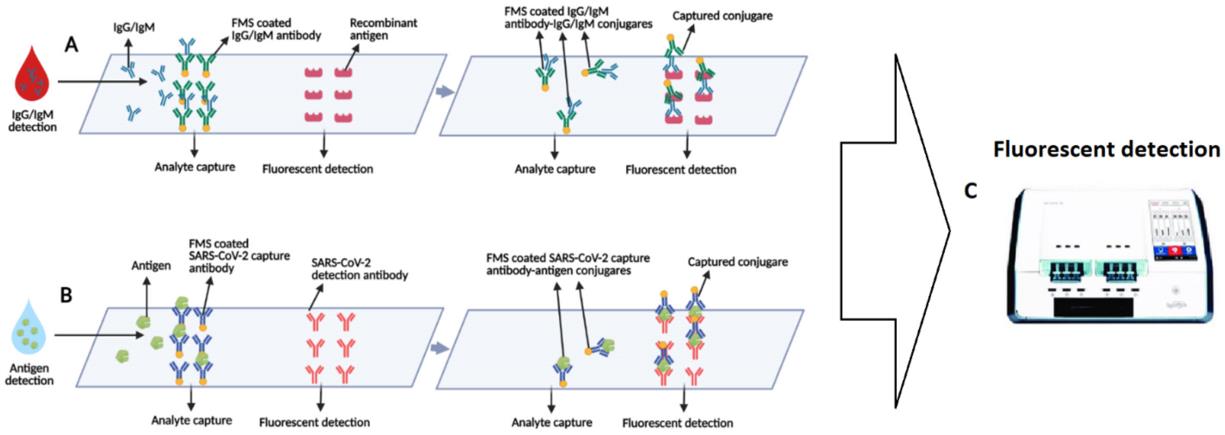 Fig.1 Schematic of the microfluidic fluorescence immunoassay for simultaneous detection of the antibodies and antigens.1
Fig.1 Schematic of the microfluidic fluorescence immunoassay for simultaneous detection of the antibodies and antigens.1
Following the sequence of TOR2, a 60-mer oligonucleotide microarray was successfully designed and implemented for the detection of SARS-CoV in the patient samples. Due to the quick and unexpected mutations in the SARS-CoV, a new microarray technique to detect 24 SNP mutations amidst the gene encoding the spike (S) protein of SARS-CoV was developed, having 100% accuracy in detection of the sample. The foremost care should be taken that the diagnostic methods are efficient enough for the broad-spectrum detection of coronavirus and can be easily deployed near/at POC, as there might be a sudden CoV outburst. The efficiency and sensitivity of this array were somewhat similar to that of the real-time RT-PCR. The evaluation of the MAP (mobile analysis platform) which is based on the microarray chip, is a new, near-POC, and compact diagnostic platform. Its efficiency in the detection of viruses was remarkable.
Our Service
In the context of the global pandemic of COVID-19, SARS-CoV-2 coronavirus has become the top topic in research. Due to its high throughput and accuracy, antigen microarray detection has great potential to play a key role in this field. As a global-leading CRO company, Creative Biolabs is professional in providing antigen microarray detection services for SARS-CoV-2 coronavirus. With advanced facilities, up-to-date technology, and experienced experts, we are confident to satisfy every customer.
| SERVICE | SAMPLE TYPE | DELIVERY | LEAD TIME |
|---|---|---|---|
| Antigen Microarray Detection for SARS-CoV-2 coronavirus | Plasma, serum, antibody, cerebrospinal fluid, urine, and saliva | Project Report; Experiment Data; Heatmap | 2-3 week |
If you are interested in our services or you have any other requirements for antigen microarray detection, please feel free to contact us for more information.
Highlight
- High throughput, rapid, and accurate detections.
- Remarkable cost performance and efficiency.
- Broad-spectrum detection of coronavirus.
- Reliable and detailed data delivery.
FAQs
-
Q1: How to achieve broad-spectrum detection of coronavirus?
A: A fancy microarray technique is applied here to detect multiple SNP mutations of the gene encoding the spike (S) protein in SARS-CoV-2 to deal with unexpected and quick mutations of SARS-CoV-2 coronavirus, making it possible for broad-spectrum detection of coronavirus. -
Q2: What kind of results can I get from this service?
A: You will receive an exhaustive project report and original experiment data within a short turnaround time of 2-3 weeks.
Resource
Reference
- Shaffaf, Tina, and Ebrahim Ghafar-Zadeh. "COVID-19 diagnostic strategies part II: protein-based technologies." Bioengineering 8.5 (2021): 54.
Related Services:
- Autoantigens General Survey Detection
- Brain and Central Nervous System Disorders Detection
- Cancer and Neoplasms Detection
- Common Allergens Detection
- Autoimmunity, Allergy, and Infection Detection
- Coronavirus-Associated Autoimmunity Detection

