- Home
- Resources
- Knowledge Center
- Reviews
- Linkers in Antibody-Drug Conjugates
Linkers in Antibody-Drug Conjugates
As the central engineering component in antibody-drug conjugates (ADCs), the bioconjugation linker fulfills dual functional imperatives through precision chemical design: maintaining covalent stability between monoclonal antibodies (mAbs) and therapeutic payloads during systemic circulation (pH 7.4, 37°C), while enabling tumor microenvironment-responsive activation via enzymatic cleavage or pH-dependent hydrolysis mechanisms. This molecular bridge's controlled dissociation kinetics must balance paradoxical requirements—achieving >95% plasma stability over 72 hours to prevent off-target toxicity versus <30-minute payload release kinetics post-internalization—parameters that directly determine clinical efficacy and therapeutic index. Modern linker development leverages computational protein engineering and tumor biology insights to create stimulus-responsive designs, particularly focusing on cathepsin-B cleavable dipeptide systems and β-glucuronidase-sensitive hydrophilic linkers, which now demonstrate site-specific conjugation efficiencies exceeding 85% with <5% premature release rates. These advancements, coupled with next-generation payloads requiring tailored release profiles, position linker optimization as the critical determinant in expanding ADC applications to solid tumors and overcoming multidrug resistance mechanisms, ultimately enabling dose reductions of 40-60% compared to conventional chemotherapy while maintaining >90% target cell cytotoxicity.
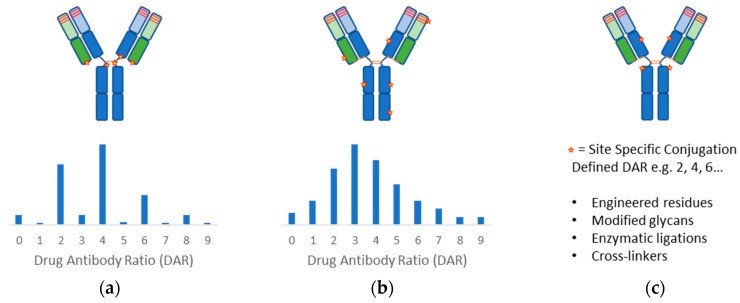 Fig 1. Antibody conjugation methods.1, 3
Fig 1. Antibody conjugation methods.1, 3
Antibody-Drug Conjugate (ADC) Linkers
ADCs are highly engineered drugs with three distinct components: a targeting antibody that home in and bind to proteins on the surface of cancer cells, an extremely potent cytotoxic medication, or payload, which kills the cells, and a linker molecule with a special chemical function to couple the antibody to the payload. The antibody is the homing device, which delivers the ADC to tumors, while sparing normal tissues. Once injected, the linker acts as a safety switch that allows the cytotoxic payload to be securely attached to the ADC in the bloodstream but only to release its deadly contents inside of cancer cells. This targeted delivery system—referred to oftentimes as a "lock and key" model—is what gives ADCs the greatest killing power against cancers while causing minimal bystander destruction characteristic of standard chemotherapy. The alchemy of ADCs is one of targeted delivery.
Chemical Triggers of the Linker
As a critical structural component of antibody-drug conjugates (ADCs), the linker not only serves as the molecular fragment enabling covalent conjugation between antibodies and small-molecule effector units, but also functions as a core element with tunable design properties. Though seemingly straightforward, this molecular architecture embodies sophisticated engineering considerations, extending far beyond a simple bridging element between antibodies and payloads in ADCs. The linker achieves ADC's targeted drug delivery mechanism through dual functional regulation: maintaining drug stability during systemic circulation while ensuring payload-specific release within target cells. Furthermore, it significantly modulates the overall physicochemical properties of the conjugated drug. Optimal linker design must satisfy two critical parameters: demonstrating high stability in the circulatory system and enabling efficient payload release following antibody-mediated endocytosis.
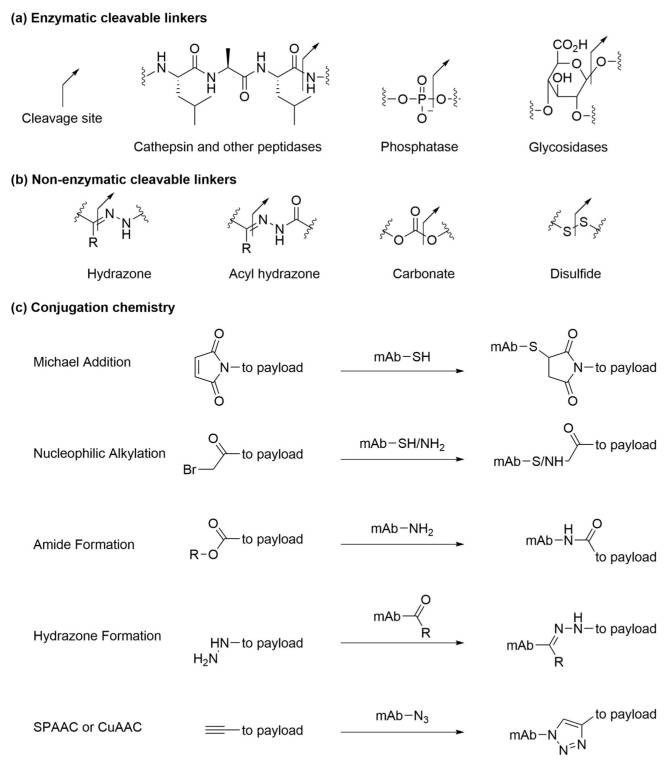 Fig 2. Different linkers in ADC drugs.1, 3
Fig 2. Different linkers in ADC drugs.1, 3
The linker inside ADCs has two critical roles to execute: attaching the antibody covalently to the cytotoxic payload and allowing cleavage for release of the toxin at targeted tumor sites. These two roles dictate two critical characteristics that the linker must possess. First, it must be extremely stable while it is in systemic circulation. Second, it must deliver precise release of active payload components at tumor sites. These seemingly opposing requirements pose significant challenges in linker design, and design of this molecule is particularly critical. The key considerations in linker design revolve around three primary concerns: the chemical groups responsible for linker cleavage, conjugation sites between linker and antibody, and attachment sites bridging linker to cytotoxic payload.
The linker addition should not induce aggregation nor compromise acceptable pharmacokinetic (PK) profiles while satisfying two fundamental requirements: being stable to prevent premature payload release in plasma and enabling efficient release of active molecules at the target site. Linkers are typically classified as cleavable or noncleavable, with distinct mechanisms of action, therapeutic advantages, and inherent limitations. These molecular bridges are a primary determinant of the pharmacokinetic profile, tumor selectivity, and therapeutic index of ADCs.
Enzymatic Cleavable Linkers
Linkers containing disulfide bonds belong to this category, where nucleophilic attack by thiol groups triggers payload liberation. These disulfide-based systems are predominantly employed with maytansinoid-class cytotoxic agents. The reactivity of disulfide bonds can be modulated through steric hindrance strategies – α-methyl substitution notably alters reduction kinetics and resistance to thiol-disulfide exchange. While this technology has been implemented in clinically approved therapeutics, its plasma stability and systemic delivery efficiency require further optimization, demonstrating inferior performance compared to newer-generation cleavable linker architectures.
To achieve controlled payload release prior to cellular internalization while minimizing extracellular degradation, lysosomal proteolytic machinery presents a strategic focus for identifying enzymes capable of mediating ADC catabolism, particularly those exhibiting elevated intracompartmental concentrations. Cathepsin B, a cysteine protease predominantly localized in late endosomes and lysosomes of mammalian cells, demonstrates marked overexpression across numerous cancer types. Pioneering studies employing cleavable dipeptide motifs as cathepsin B substrates established structure-activity relationship (SAR) principles for doxorubicin prodrug design, providing critical insights into dipeptide sequence optimization for enzymatic activation.
Phosphatase- and Pyrophosphatase-Cleavable Linkers
Capitalizing on the lysosome-specific expression of hydrolytic enzymes such as phosphatases and pyrophosphatases, these linkers incorporate phosphate or pyrophosphate groups. Enzymatic hydrolysis of these moieties triggers payload release.
β-Glucuronidase- and β-Galactosidase-Cleavable Linkers
This design integrates enzyme-specific hydrolysable groups (e.g., glucuronic acid or galactose) paired with self-immolative spacers. Following hydrolysis of β-glucuronide or β-galactoside groups by their respective enzymes, the spacer undergoes spontaneous degradation to liberate the payload.
Sulfatase-Cleavable Linkers
Employing a similar rationale, these linkers incorporate sulfated esters combined with self-immolative spacers. Sulfatase-mediated cleavage of the sulfate group initiates spacer autodegradation, enabling toxin release.
Acid-Labile Linkers
Leveraging the acidic tumor microenvironment (pH <6 vs. physiological pH ~7.4), these systems utilize pH-sensitive bonds such as hydrazones or carbamates. These linkages remain stable under neutral conditions but undergo selective cleavage in acidic milieus to release cytotoxic agents.
Non-enzymatic Cleavable Linkers
Non-cleavable linkers demonstrate inherent inertness to common chemical and enzymatic environments in vivo. For ADCs employing non-cleavable linkers, internalization is mandatory, requiring subsequent degradation of the antibody component by lysosomal proteases to liberate the active payload. Several non-cleavable linkers have been explored in ADC development, with the most representative being SMCC, as utilized in the therapeutic agent. Such linkers exhibit particular suitability for small molecules tolerant of structural modifications—specifically when the pharmacophore remains distal to the conjugation site. However, payloads conjugated via these linkers typically lack bystander effect capability due to the limited membrane permeability of released catabolites. Current pharmaceutical research prioritizes cleavable linker systems, though non-cleavable platforms remain valuable for specific therapeutic contexts requiring precise spatial control of payload activation.
Challenges and Innovations in Linker Development
Designing effective linkers for ADCs faces a problem—they must balance stability and fragility perfectly. If too stable, like the failed drugs, the linker resists breaking even in tumors, rendering the drug useless. If too fragile, toxic payloads leak into the bloodstream, harming healthy tissues. Compounding this challenge is tumor diversity: variations in pH, enzyme levels, and reducing environments between cancers mean a linker optimized for breast cancer might fail in pancreatic tumors. Additionally, ultra-potent drugs like pyrrolobenzodiazepines demand exceptionally stable linkers to prevent lethal leaks, further narrowing the design window.
To address these hurdles, scientists are pioneering precision-driven strategies. Site-specific conjugation techniques, such as THIOMAB, replace random drug attachment with engineered cysteine residues, ensuring uniform linker placement and enhanced stability. Meanwhile, AI tools from companies like Absci accelerate linker optimization by predicting how chemical modifications impact ADC behavior. Perhaps most promising are "smart" linkers activated only by tumor-specific triggers. For instance, Stanford researchers designed a linker requiring two cancer-associated enzymes to release its payload, virtually eliminating off-target effects in preclinical trials. These advances highlight a shift toward linkers that act as molecular "logic gates," unlocking chemotherapy's power exclusively within tumors.
The Future Hinges on Linker Innovation
Regulatory data reveal that 10 ADCs have received FDA approval during the current quinquennium, or around 4% of all new license therapeutics in this period. Thorough preclinical research regularly emphasizes the leading role of optimized linker systems as important determinants of ADC success. A good linker topology must simultaneously satisfy two fundamental demands: systemic circulatory stability under circulation and intracellular payload release with specific spatiotemporal regulation. In view of the profound influence of linker chemistry on conjugate stability, toxicity profile, pharmacokinetic activity, and pharmacodynamic activity, judicious evaluation of linker selection is an essential step in ADC development. Furthermore, linker design must also involve systematic evaluation of reactive sites on cytotoxic molecules, including both drug-derived functional groups and monoclonal antibody conjugation sites.
New research areas are focused on new linker modalities, including photoactivatable ADC bridges and bioorthogonal cleavage systems. Although these newer platforms hold theoretical advantages like enhanced target specificity, enhanced potency, and reduced off-target toxicity, their transfer to clinic remains in its early stages due to issues of technical maturation and in vivo stability validation that are yet to be resolved. Continued evolution of next-generation linker technology will offer a solution to further extend the ADC design platform, potentially increasing the therapeutic ratio and clinical utility of these targeted oncology drugs.
Creative Biolabs provides specialized linker synthesis services for ADC development, targeting diverse payload release mechanisms.
- pH-Sensitive Linkers: Acid-labile (hydrazones) release payloads in tumor acidic microenvironment (pH 5.0–6.5), ideal for solid tumors.
- Disulfide Linkers: Reductive cleavage via glutathione releases payloads intracellularly, enhancing circulation stability.
- Peptide Linkers: Enzyme-cleavable (Val-Cit) by cathepsins, enabling specific payload release and modular design.
- β-Glucuronide Linkers: β-Glucuronidase-mediated hydrolysis triggers release in necrotic regions, reducing off-target toxicity.
- Non-Cleavable Linkers: Stable until lysosomal degradation, suitable for highly internalizing antigens to minimize premature release.
Services ensure controlled release, stability, and target specificity, supporting ADC development from research to preclinical stages.
References
- Leung D, Wurst JM, Liu T, et al. Antibody Conjugates-Recent Advances and Future Innovations. Antibodies 2020, 8;9(1): 2. https://doi.org/10.3390/antib9010002
- Fu Z, Li S, Han S, et al. Antibody drug conjugate: the "biological missile" for targeted cancer therapy. Signal transduction and targeted therapy 2022, 7(1): 93. https://doi.org/10.1038/s41392-022-00947-7
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only. NOT FOR CLINICAL USE.

Online Inquiry
Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
