- Home
- ADC Development
- DrugLnk™ Custom Linker-Payload Synthesis
- Linker Module Synthesis
- Disulfide Linker Synthesis
Disulfide Linker Synthesis Service
Creative Biolabs has extensive professional expertise in providing customarily designed payload-linker complexes for antibody drug conjugate (ADC) development. With state-of-art equipment, advanced techniques, as well as the "DrugLnk" organic synthesis platform, scientists from Creative Biolabs are dedicated to design and synthesize payload-linker complexes containing disulfide linkers to fulfill clients’ requirements in a highly productive and cost-effective way.
Disulfide linkers (sometimes refered to as glutathione-sensitive linkers) are a family of chemically cleavable linkers that non-selevtively release the payload drug upon the exposure to an altered chemical environment, which in this case, is the higher reducing potential within the tumor cells compared to that of the criculating plasma.
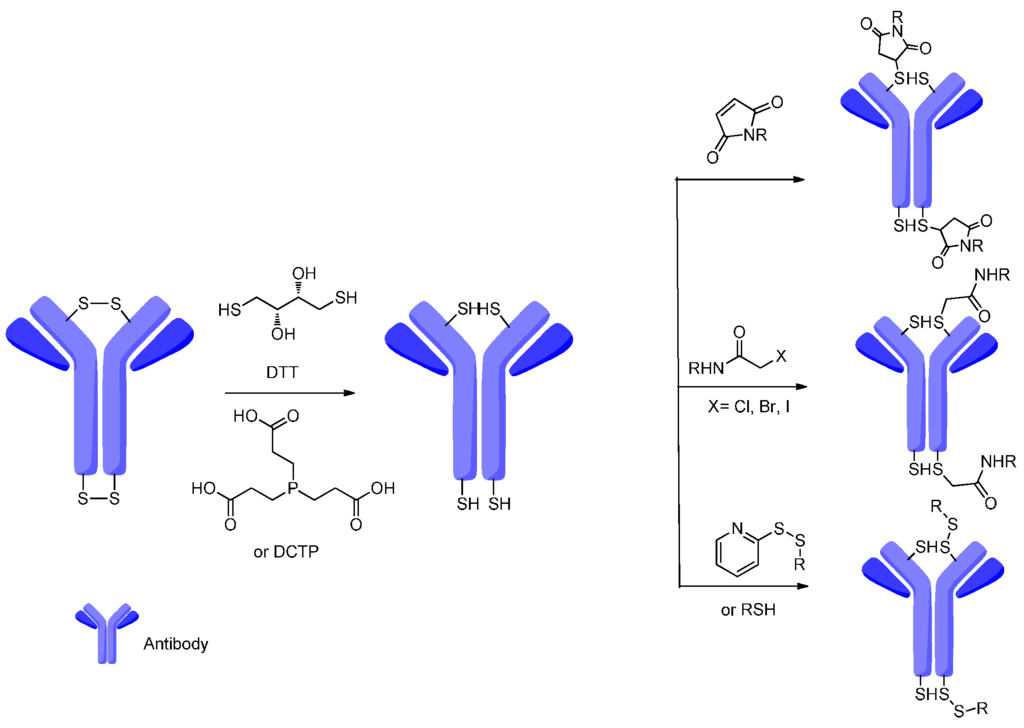 Fig.1 Schematic representation of conjugate with a disulfide linker.1,3
Fig.1 Schematic representation of conjugate with a disulfide linker.1,3
Disulfides are thermodynamically stable at physiological pH and are designed to release the conjugated payload drug upon internalization into the tumor cells, where the cytosol provides a much stronger reducing environment compared to the extracellular environment (blood circulation). The scission of disulfide bridge requires the presence of a cytoplasmic thiol co-factor, such as (reduced) glutathione (GSH) or the intracellular enzyme protein disulfide isomerase. In many occasions, for ADCs containing disulfide linkers, lysosomal processing is necessary for drug activation. For these ADCs, the disulfide-linked drugs are first liberated intact by proteolytic degradation of the antibody and then released as active metabolites through disulfide exchange or by reducing agents such as glutathione inside the cells. Compared with pH-sensitive (acid labile) linkers such as hydrazones, disulfied linkers exert enhanced stability in circulation, reduced off-target drug release/toxicity, and selective drug release in the cytosol. What’s more, the serum stability of disulfide linkers can be further improved by introducing hinderance groups near the disulfide cleavage sites.
Many payloads for ADC development can be conjugated to an antibody via disulfide linkers. Common payloads for this application include calicheamicin, taxoids, and maytansinoid. Maytansinoid derivatives and thiol-containing maytansine analogues (DM1, DM4…) are among the most widely used payloads for current ADCs in clinical trial pipeline whose conjugation are achieved via disulfide bonds or thioether linkage in a chemically cleavable or noncleavable manner to an antibody, respectively.
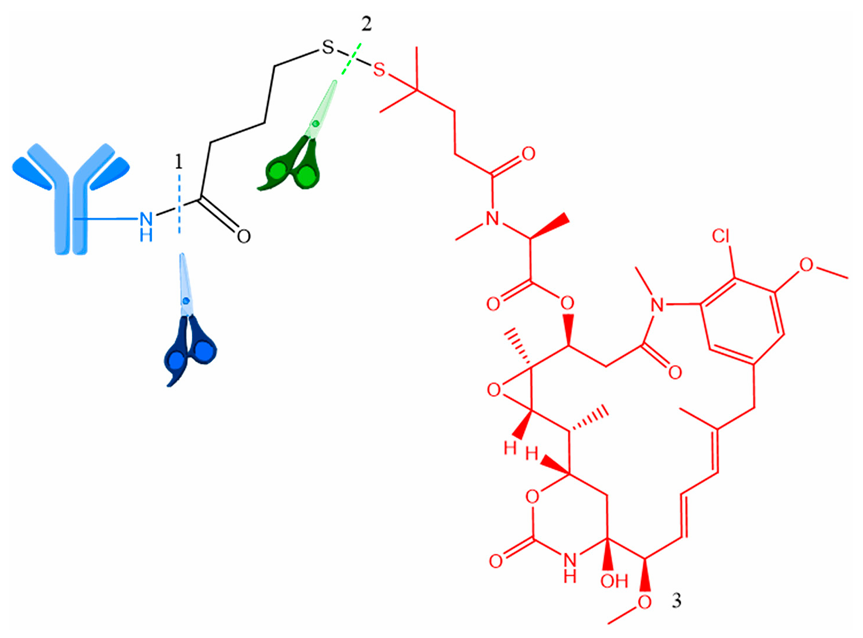 Fig.2 ADCs containing a disulfide linker.2,3
Fig.2 ADCs containing a disulfide linker.2,3
To fulfill customers’ specific demands, Creative Biolabs has developed the "DrugLnk" platform and we are dedicated in providing customized design services using disulfide linkers for ADC developments. Depending on the antibody, payload drug, and tumor target, we will design or select the most suited linker to achieve targeted drug delivery. In the meantime, we also provide other services for the benefit of ADC development. Please feel free to contact us for more information and a detailed quote.
References:
- Lu, Jun, et al. "Linkers having a crucial role in antibody–drug conjugates." International journal of molecular sciences 17.4 (2016): 561.
- Chis, Adriana Aurelia, et al. "Antibody–Drug Conjugates—Evolution and Perspectives." International Journal of Molecular Sciences 25.13 (2024): 6969.
- Distributed under Open Access License CC BY 4.0, without modification.
For Research Use Only. NOT FOR CLINICAL USE.

Online Inquiry
Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
