- Home
- Resources
- Knowledge Center
- Reviews
- Cytotoxic Payloads of ADCs
Cytotoxic Payloads of ADCs
The ADC drug theory was first envisioned by German Nobel winner Paul Ehrlich in 1913. But only with the innovation of hybridoma technology to make monoclonal antibodies in 1975 did the era of ADC development really begin. ADCs were developed as a treatment approach due to the limited availability and high cost of novel biological treatments (e.g., cellular therapies and immunotherapies) and the unsatisfactory clinical activity and severe toxic side effects of conventional chemotherapy. By engaging tumor-bound antibodies with cytotoxic loads, ADCs achieve selective and potent killing of tumor cells with enhanced safety profiles. Characterized by fewer side effects, expanded therapeutic applications, and bigger therapeutic ratios, ADCs have emerged as one of the fastest-growing categories of drugs within oncology in recent years.
Importance of the ADC Payload
Mode of action involves antigen recognition-dependent internalization of ADC via endocytosis. Following lysosomal breakdown, the active payload is intracellularly released to induce cytotoxicity effects that ultimately kill cancer cells. The intracellular payload amount is determined by three variables: cell surface antigen density, drug-to-antibody ratio (DAR), and kinetics of antigen recycling. Most importantly, released payloads may either demonstrate salutary bystander effects through membrane permeability (killing adjacent tumor cells) or, potentially, systemically cause toxicity through untargeted spread.
Function of ADC Payloads
The cytotoxic element of antibody-drug conjugates (ADCs), sometimes referred to as their core therapeutic agent, executes targeted elimination of malignant cells through specialized biochemical pathways. This biologically active component remains inactive during circulation, only manifesting its cell-killing properties after successful delivery into tumor environments.
Key Functional Pathways
Genetic Material Disruption: Agents such as calicheamicin derivatives induce structural damage to DNA through cross-linking mechanisms, while compounds like duocarmycin analogs modify nucleic acid integrity. This interference with genetic replication processes activates programmed cell termination pathways.
Cytoskeletal Interference: Specific microtubule-targeting substances including monomethyl auristatin E (MMAE) and maytansine derivatives impede essential cellular architecture reorganization. By preventing proper chromosomal segregation during mitosis, they effectively terminate cell proliferation cycles.
Enzyme Complex Stabilization: Certain synthetic compounds modeled after exatecan interact with DNA processing machinery. Through persistent stabilization of transient DNA-protein complexes, they induce lethal replication errors during cellular division cycles.
These specialized therapeutic agents demonstrate exceptional potency, requiring minimal concentrations (picomolar range) to achieve therapeutic effects. This characteristic enables effective targeting of malignancies with limited biomarker expression. Unlike conventional cytotoxic treatments, the conditional activation mechanism-dependent on cellular internalization-significantly minimizes off-target effects on healthy tissues, addressing a critical challenge in traditional oncology therapies.
Importance and Characteristics of the ADC Payload
The clinical efficacy of ADC drugs is intrinsically determined by the bioactivity and molecular characteristics of their cytotoxic payloads, and the mode of action of the payload is a principal determinant of drug action. The ideal payload molecule should meet a number of demands simultaneously: First, the molecule needs to exhibit ultrapotent cytotoxic activity to transcend biological constraints like low expression of target antigen in solid cancers, inadequate tissue penetration by antibodies, and ineffective endocytosis, resulting in tumor killing despite low intracellular accumulation levels. Second, it demands aggressive mitigation of immunogenicity risk—though with humanized antibodies and synthetic delivery payloads, there is still residual immunoactivation risk compared to traditional antibody treatments, particularly through protein elements that are capable of triggering immune reactions sufficient to undermine therapeutic effect or induce significant adverse events. Moreover, utilization of a molecular streamlining strategy achieves twofold optimization by reducing immunorecognition vulnerability as well as optimizing tumor penetration potentiality, a design principle of high importance for expanding the therapeutic window through increased tumor selectivity and systemic security profiles.
The ADC load should have high stability. Due to the long half-life of antibodies in circulation, ADCs should remain stable in the blood circulation to avoid release or decomposition. The payload should also remain stable in the cytoplasm and lysosomes and not be significantly degraded at low pH. ADC payloads should have functional groups that can be modified without significantly affecting their effectiveness. The payload must have a modifiable functional group or site where it can be conjugated to the monoclonal antibody. The modification sites must be carefully selected to preserve the efficacy of the parent drug. More importantly, when non-cleavable linkers are used, the payload must retain its effectiveness even after antibody degradation.
ADC payloads should demonstrate non-target collateral cytotoxicity. Mechanistically, certain ADC agents undergo cellular internalization followed by liberation of low-molecular-weight, non-polar, membrane-permeant hydrophobic compounds. These bioactive entities diffuse across cellular membranes, exerting cytotoxic effects on adjacent tumor cells lacking antigen expression—a phenomenon termed "paracrine cytotoxic spread." This biological process holds critical therapeutic value for neoplasms with heterogeneous antigen distribution patterns. Therapeutic payloads demonstrating capacity for paracrine cytotoxic spread are preferentially employed in malignancies exhibiting heterogeneous or low-level target antigen presentation. The bystander mechanism fundamentally enhances drug penetration depth and compensates for incomplete antigen targeting, particularly in solid tumors with complex microenvironmental barriers. This strategic selection enhances treatment efficacy against tumors with incomplete target coverage while maintaining spatial precision within malignant tissue microdomains.
Finally, the ADC payload should have appropriate water solubility. The payload must have appropriate water solubility to promote conjugation with the antibody and ensure sufficient solubility of the conjugate under physiological conditions. When excessive hydrophobic payloads are conjugated to antibodies, the resulting ADCs tend to aggregate and become unstable. In addition, the hydrophilicity of the payload affects the cell membrane permeability of the parent ADC or its metabolites, thereby affecting its bystander killing activity. Also, the payload should be able to enter the cell. Because most ADCs need to enter tumor cells to release their payload. For example, many payloads targeting cell membranes cannot meet this requirement.
Various Payloads of ADC
Maytansine Derivative
Maytansine represents a highly potent microtubule assembly inhibitor capable of inducing mitotic arrest in cells. However, its structural complexity poses conjugation challenges due to the absence of reactive functional groups. To address this limitation, potent derivatives incorporating SMe-containing moieties were engineered through strategic molecular modifications. The pioneering examples of this class include DM1 and DM4, which feature methiopropionyl substituents replacing the native N-acetyl groups. This structural optimization achieves dual objectives: preserving cytotoxic potency while introducing conjugation-compatible sites, thereby enabling effective antibody linkage through thiol-maleimide chemistry. The modified derivatives maintain comparable antimitotic activity to the parent compound while resolving the critical conjugation bottleneck, demonstrating IC50 values in the picomolar range against various cancer cell lines. This molecular redesign exemplifies rational drug engineering to overcome pharmacological limitations while enhancing therapeutic applicability in targeted cancer treatment paradigms.
Microtubules targeting payloads
Microtubules are the essential structural components of the eukaryotic cytoskeleton that regulate essential cellular processes including morphological integrity, signal transduction, organelle transport, locomotion of cells, and mitosis. Microtubules' contribution to organizing the mitotic spindle renders them a target of preference in oncology. Microtubule-targeting agents operate with antimitotic efficacy by interfering with the spindle apparatus and are demonstrated to be preferentially cytotoxic for rapidly proliferating malignant cells as opposed to resting normal tissues-a biological selectivity that justifies their dominance as ADC payloads. Microtubule inhibitors top the clinical ADC development pipelines as second-generation cytotoxic warheads. A few research consortia have made structural optimization attempts to enhance their pharmaceutical quality.
EG5 inhibitors
Spindle kinesin is an ATP-dependent movement protein involved in the separation of centrosomes in the cell cycle. Therefore, blocking this important event in mitosis with KSP inhibitors can produce antitumor efficacy.
DNA damage drugs
Ducamycin is a powerful cytotoxic substance that binds to small grooves in DNA through its highly active cyclopropane ring and alkylates adenine at the N3 position. The cytotoxic activity of the non-cyclized, halomethyl form of ducamycin was significantly reduced. Since the phenol group in the molecule can act as a mesomer activator, thereby forming electrophilic cyclopropane, the ligation strategy in the development of ducamycin ADC focuses on linker ligation of phenol functional groups.
Payload Diversification of ADC
The initial generation of ADCs employing conventional chemotherapeutic agents demonstrated limited therapeutic efficacy stemming from suboptimal cytotoxic potency of payloads coupled with suboptimal intracellular delivery efficiency—a biological limitation exacerbated by the fact that only a minimal fraction of administered conjugates successfully delivered their payloads into target cells. The pioneering payload arsenal primarily comprised two mechanistic classes: microtubule-disrupting agents and DNA-alkylating compounds such as calicheamicins. For decades, these microtubule-targeting and DNA-intercalating agents dominated ADC development pipelines. Contemporary research however is expanding the payload spectrum through innovative modalities including heat shock protein 90 inhibitors, translation termination disruptors, and proteasome-targeting molecules. This diversification initiative has catalyzed the preclinical advancement of unconventional antibody conjugates featuring payloads with novel mechanisms of action, particularly those targeting non-proliferative cellular processes and tumor microenvironment modulation. Strategic payload innovation now focuses on overcoming multidrug resistance mechanisms while enhancing tumor selectivity through microenvironment-responsive activation paradigms and epigenetic modulation capabilities.
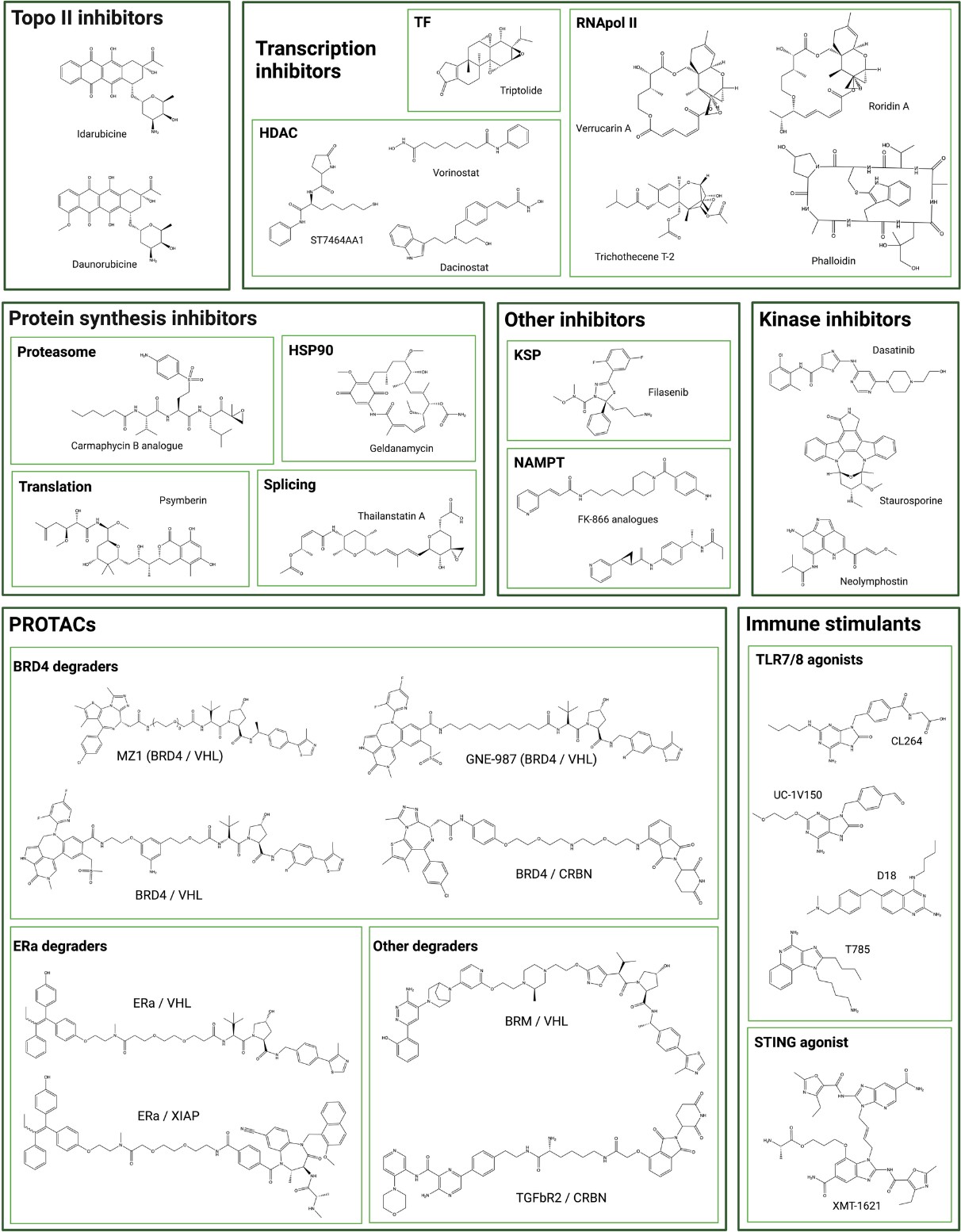 Fig. 1 Chemical structure of unconventional ADC payloads conjugated.1, 2
Fig. 1 Chemical structure of unconventional ADC payloads conjugated.1, 2
ADC payloads are key to the success of precision cancer therapy. Their evolution—through the move away from conventional chemotherapeutics to highly tailored molecular agents—dramatizes their critical role in amplifying patient gain. Present emphasis lies in enhancing payload diversity, delivery specificity, and evasion of drug resistance, making ADCs shining stars of precision medicine. With payload development gaining momentum, hope that ADCs will be able to cure recalcitrant cancers and reduce treatment toxicity grows geometrically, heralding a new era in oncology.
Creative Biolabs, as the leader in ADC development, provides comprehensive payload synthesis services
- Microtubule toxin synthesis: tubulysins, auristatins, etc., inhibit tubulin polymerization, used in solid tumors and multidrug resistant cancers, purified by Fmoc SPPS and RP-HPLC.
- DNA toxin synthesis: calichemycin analogues, etc., induce DNA double strand breaks, are suitable for hematological malignancies and DNA repair deficient tumors, supporting gene synthesis and oligonucleotide modification.
- Transcriptional toxin synthesis: amatoxins, thailanstatin A, blocks RNA polymerase II, targets MYC-driven tumors, has high selective cytotoxicity.
- Inhibitor synthesis: Covering six types of kinase inhibitors (such as EGFR, BTK), optimizing ADC specificity, and being used in cancers related to signaling pathways such as PI3K/AKT/mTOR.
References
- Fu, Zhiwen, et al. "Antibody drug conjugate: the "biological missile" for targeted cancer therapy." Signal transduction and targeted therapy 7.1 (2022): 93. https://doi.org/10.1038/s41392-022-00947-7
- Conilh, L., Sadilkova, L., Viricel, W. et al. Payload diversification: a key step in the development of antibody–drug conjugates. J Hematol Oncol 2023, 16, 3. https://doi.org/10.1186/s13045-022-01397-y
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only. NOT FOR CLINICAL USE.

Online Inquiry
Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
