- Home
- ADC Development
- DrugLnk™ Custom Linker-Payload Synthesis
- Drug Module Synthesis
- Transcription Toxin Synthesis
- Amatoxin Synthesis
Amatoxin Synthesis Service
With a comprehensive understanding and extensive experience in antibody-drug conjugate (ADC) developments, Creative Biolabs offers highly customized ADC design and preparation using amatoxin and amatoxin analogs as payloads.
Gene transcription is a process that transfers the genetic information into carriers such as mRNA and guides subsequent cellular metabolic activities. Interruption of gene transcription will result in catastrophic impacts and lead to cell death. Many of the transcription inhibitors have decent pharmacological effects against tumor growth and a number of anti-cancer drugs have been identified or developed to restrain transcription, among which amatoxins have been shown to exhibit high tumor inhibition potency.
Amatoxins are a group of toxic compounds commonly produced by green and white Amanita muscaria and result in fatal human Amanita intoxications upon ingestion. They are synthesized via the cleavage, modification, and cyclization of precursor peptides that usually contain a ~10 amino acid-residue leader peptide followed by the amatoxin peptide and a recognition sequence. The recognition sequence starts with a conserved Cys residue and has several conserved peptide motifs including the DDV motif and the LLTRGE motif. Chemically, amatoxins are cyclic octapeptides with a basic peptide sequence template: Ile-Trp-Gly-Ile-Gly-Cys-Asn-Pro. This octapeptide is cyclized by the peptide bond between Ile and Pro residues and it is also featured by an internal cross-bridge formed by the Cys and Trp residues. Modifications (mainly hydroxylation) to some side chains within the template peptide (4-hydroxyPro, γ, δ-dihydroxyIle, and 6-hydroxyTrp) further diverse the chemical complexity of amatoxins.
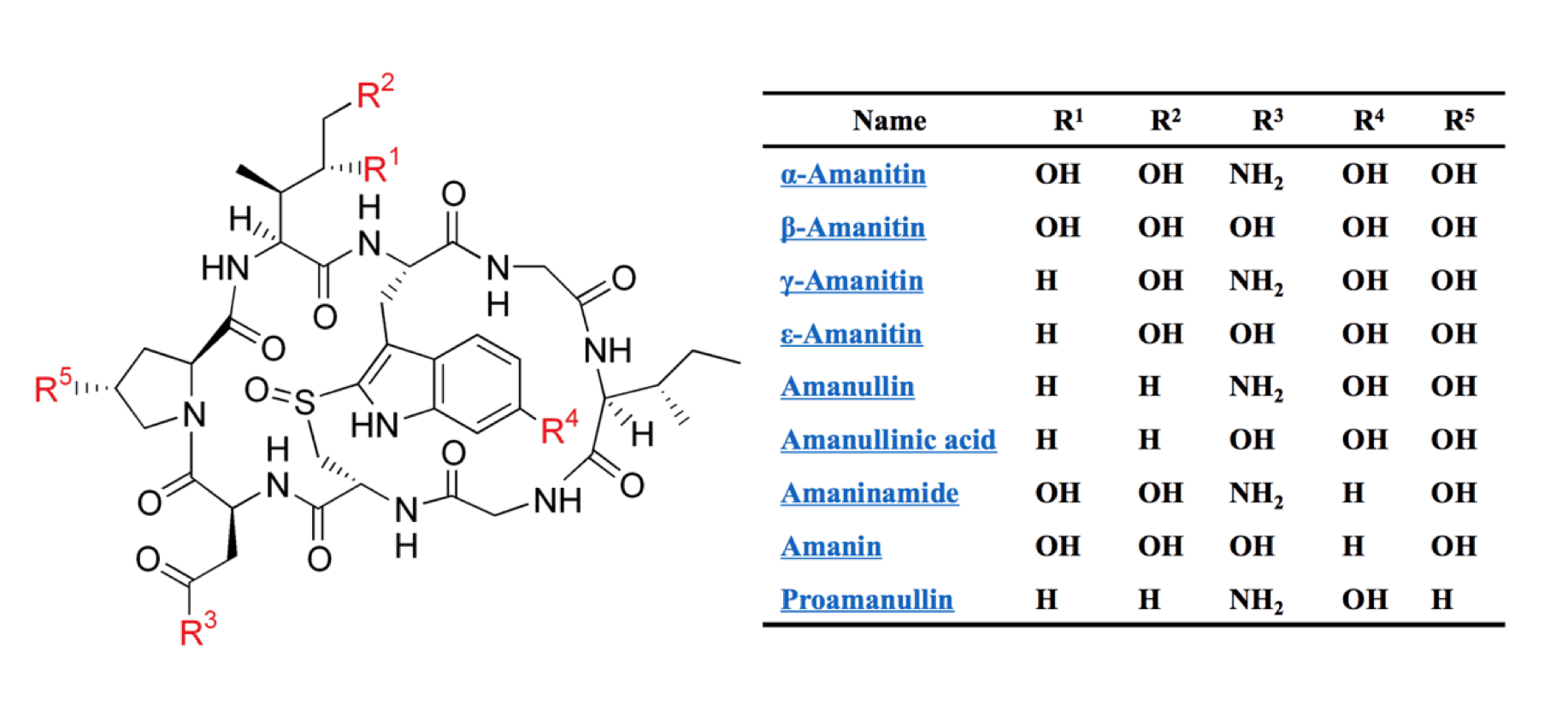 Fig.1 Cyclic octapeptide structure of amatoxin and its variants.1
Fig.1 Cyclic octapeptide structure of amatoxin and its variants.1
Amatoxins Mode of Action (MOA)
Amatoxins are powerful inhibitors of gene transcription and preferentially target RNA polymerase II, a crucial enzyme in mRNA biosynthesis. RNA polymerase II complex contains a conserved 35-amino-acid-long bridge helix that is important for the DNA translocation. α-amanitin, the major form of amatoxin, has been demonstrated to bind the free RNA polymerase II core adjacent to the bridge helix and thus interfere with its movement and lead to the diminishing of DNA translocation during mRNA synthesis. In the meantime, structural analysis of α-amanitin with RNA polymerase II elongation complex also revealed that α-amanitin confines the trigger loop into a new confirmation that stabilizes the elongation complex into a translocation intermediate, hindering DNA translocation.
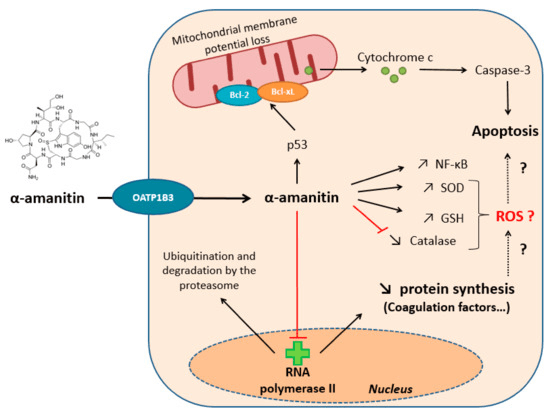 Fig.2 Amatoxins Mode of Action.2,4
Fig.2 Amatoxins Mode of Action.2,4
Amatoxins-based ADCs
Amatoxins, especially α-amanitin, are promising toxic payloads in ADC developments based on their specific MOA and high water solubility. Recently, α-amanitin based ADCs are under active development and evaluation, while some have exhibited decent efficacy in drug resistant tumor lines, for example, tumor-initiating cells and tumor cells expressing multi-drug resistant transporters.
 Fig.3 Structure of chiHEA125-Ama ADC.3,4
Fig.3 Structure of chiHEA125-Ama ADC.3,4
With our well-established "DrugLnk" organic synthesis platform, the experienced scientists here at Creative Biolabs is dedicated in the development of amatoxins-linker complexes using readily available or customized linkers for antibody conjugation in a timely and cost-effective manner. Our customarily tailored services and high quality products will contribute greatly to the success of your projects.
Creative Biolabs also provides other various services regarding ADC development. Please feel free to contact us for more information and a detailed quote.
References:
- Lyons, Mark J., Carsten Ehrhardt, and John J. Walsh. "Orellanine: From fungal origin to a potential future cancer treatment." Journal of Natural Products 86.6 (2023): 1620-1631. Distributed under Open Access License CC BY 4.0, modification-based Fig 1.
- Le Daré, Brendan, Pierre-Jean Ferron, and Thomas Gicquel. "Toxic effects of amanitins: repurposing toxicities toward new therapeutics." Toxins 13.6 (2021): 417.
- Kostova, Vesela, et al. "The chemistry behind ADCs." Pharmaceuticals 14.5 (2021): 442.
- Distributed under Open Access License CC BY 4.0, without modification.
For Research Use Only. NOT FOR CLINICAL USE.

Online Inquiry
Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
