Support
Immunohistochemistry (IHC) is a crucial auxiliary means for pathologists in routine diagnostic work and basic and clinical research as well as biomarker exploration because IHC will make sure the expression of target molecules in the context of the microenvironment. Here we summarize the general procedure of IHC. It has several key steps. This includes correct handling of specimens, proper fixation, paraffin block preparation, antigen retrieval, selection and preparation of antibodies and reagents, incubation, washing, and anti-staining.
Step 1: Tissue Handling and Fixation
Tissue fixation will rely on some ways on the kind of antigen to be detected. Formaldehyde is the gold standard fixative for routine histology and immunohistochemistry. Formalin fixation may be a progressive time- and temperature-dependent method. Fixation of tissues is necessary to 1) adequately preserve cellular components, including soluble and structural proteins; 2) prevent autolysis and displacement of cell constituents, including Ags and enzymes; 3) stabilize cellular materials against deleterious effects of subsequent procedures; and 4) facilitate conventional staining and immunostaining. Two types of fixatives are utilized in histopathology: cross-linking (noncoagulating) fixatives and coagulating fixatives. Tissues/organs should be placed in small pieces (approximately 3 mm3) immediately into 10% neutral buffered formalin and fixed for 24-48h at room temperature.
- Samples ought to be < 4 mm thick.
- Immerse immediately the sample to be fixed in 10% neutral buffered formalin for 1-2 days.
- Trim samples.
- Embed samples in paraffin.
- Cut 3-5 μm thick sections with a microtome.
- Place sections on silanized slides.
- Dry sections in a 55-60℃ oven for 30-60 min.
- Stain the sections after deparaffinizing.
Step 2: Sectioning and Storage
Although 3- to 4-μm-thick sections are generally recommended for IHC staining, the requirements are different for sections being prepared for shipping. Tissue sections for IHC staining that are prepared for shipping should be of reproducible thickness, with 2-μm-thick sections recommended. When 2-μm-thick sections are not possible, laboratories should strive to ensure that sections are no more than 4 μm in thickness.
Step 3: Antigen (or Epitope) Retrieval
Fixation with cross-linking agents modifies the tertiary and quaternary structure of many antigens making them undetectable by antibodies. Antigen retrieval (AR) methods are intended to retrieve the loss of antigenicity, theoretically returning proteins to their prefixation conformation. Approximately 85% of antigens fixed in formalin require some type of AR to optimize the immunoreactions. In this case, antigen retrieval may provide better IHC signals. The heat-induced epitope retrieval (HIER) is the most widely used antigen retrieval method at present. HIER is widely used for immunohistochemistry on formalin-fixed paraffin-embedded tissue and includes temperatures well above the melting point of paraffin. Various methods can be used for heating: microwave oven, heating plate, pressure cooker, and water bath under different conditions.
Step 4: Protein Blocking
Protein blocking step to reduce unwanted background staining. Incubation time for the procedure varies from 30 minutes to overnight. Incubation temperatures also range from 4℃ to room temperature.
Step 5: Endogenous Enzyme Blocking
3% diluted hydrogen peroxide is widely used to block endogenous peroxidase activity. Similarly, endogenous alkaline phosphatase (AP), which is prevalent in frozen tissues, should be blocked by levamisole at a concentration of 10 mM.
Step 6: Antibody Selection and Validation
In terms of validity and reliability, antibodies used in studies are generally classified into three types: well-known antibodies with well-documented evidence, well-known antibodies used in surrogate species or unvalidated tissues, and unknown antibodies that are inconsistent or undocumented. When an unknown antibody is validated, it is important to select suitable positive and negative control tissues
Step 7: Detection System
The choice of the detection system is very important because the sensitivity of immune response depends mainly on the detection system used. Commonly used detection systems include tyramine amplification system, labeled streptavidin-biotin method, polymer-based detection system, phosphatase anti-phosphatase method, and avidin biotin complex method.
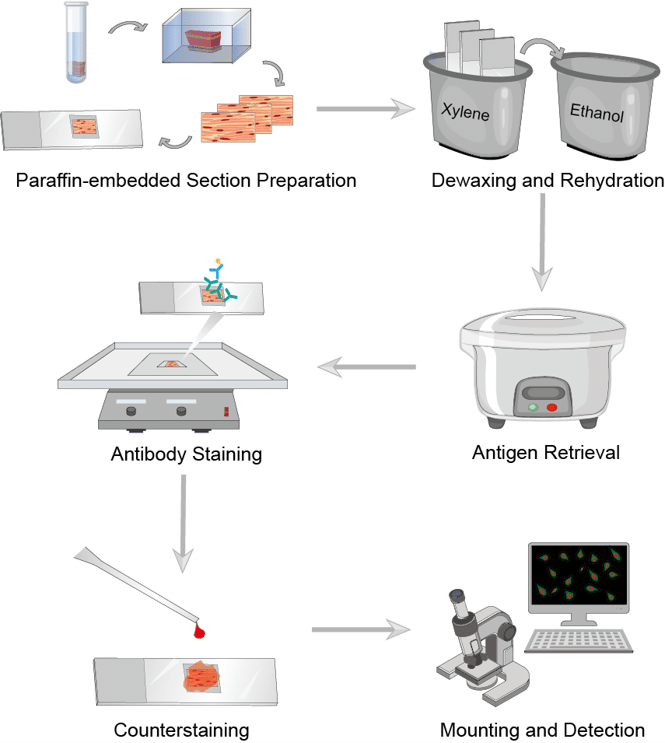 Fig.1 The workflow of IHC.
Fig.1 The workflow of IHC.
Step 8: Counterstaining
Counterstaining provides contrast to the chromogens for better discrimination of the target signal. Hematoxylin is the most commonly used counterstain, although various colors are now being used as techniques of multiplex IHC progress.
Creative Biolabs is capable to provide comprehensive products and NAA Services about NAA. Our IHC platform can give a strong support to the NAA detection services. If you are interested in our services and products, please contact us for detailed solutions.

