- Home
- UTC Development
- Fragment-Drug Conjugate Development
- Antibody Fragment-Drug Conjugate Development
- Fab-Drug Conjugate Development
- Fab-Drug Conjugation by Rebridging Method
Fab-Drug Conjugation Service by Rebridging Method
Amongst the plethora of antibody formats, Fab fragments have many advantages over other antibody fragments, including straightforward of preparation via protein expression, standard affinity chromatography purification from culture supernatant using anti-lambda, anti-kappa, or anti-CH1/CL media, high thermostability, and possession of native thiols forming a solvent accessible disulfide bond, which upon reduction can be used as attachment points for conjugation. Creative Biolabs now provides site-specific conjugation for Fab fragment-drug conjugates (Fab-based FDCs) development via disulfide bonds rebridging.
Rebridging Methods
A well-known example for conjugation is the reduction of native disulfide bonds, followed by selective conjugation of the liberated cysteine thiol groups, which is frequently accomplished by the reaction with maleimides. Other sophisticated methods make use of bifunctional reagents, which allow for bridging two thiols generated from the reduction of a disulfide bond. On one hand, bis-reactive electrophilic maleimides were used, to provide a C2-bridge. Alternatively, such rebridging of disulfide bonds could also be accomplished with double-reactive Michael acceptors to give a C1- or C3-bridge. Here, we introduce some typical ways to illustrate how to achieve homogeneous FDC products by rebridging of disulfide bonds.
Case 1: Reduction and Functional Rebridging All in One
This case, designing a reagent that could incorporate both reducing and re-bridging functions, presents a significant step towards next-generation disulfide stapling reagents. This strategy has been shown to result in a high local concentration of bridging agent, which has been exploited for the functional rebridging of a multi-disulfide system without disulfide scrambling.
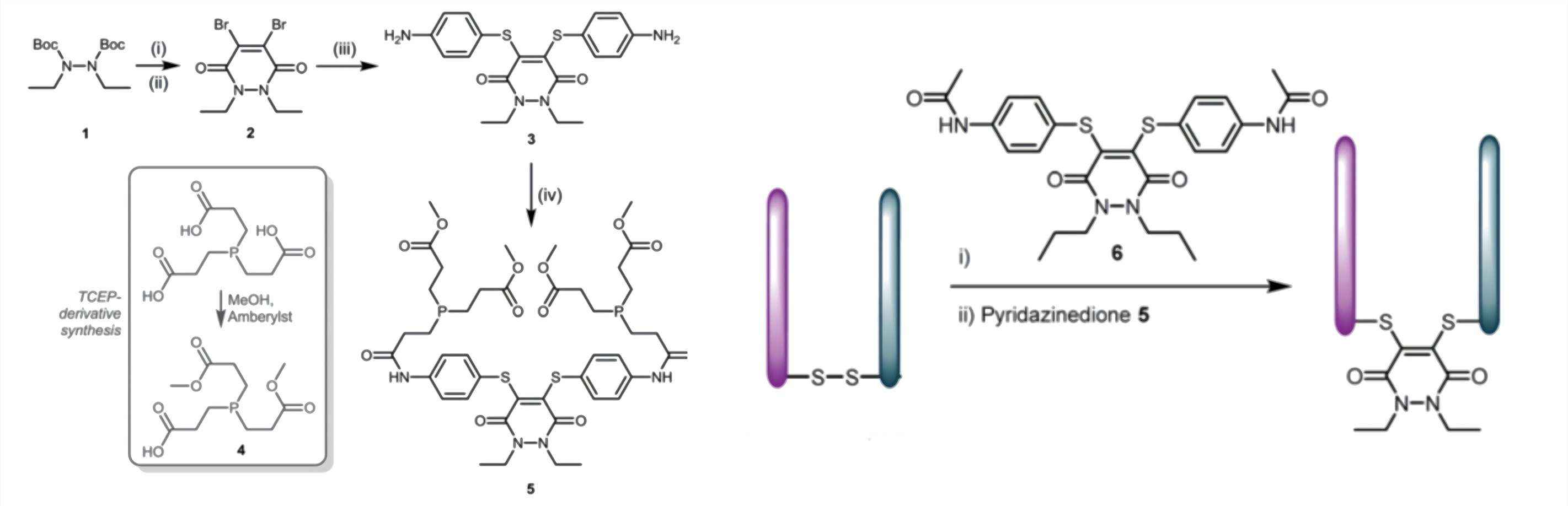 Fig.1 Synthesis of dithioaryl (TCEP) pyridazinedione and the incubation with Fab.1,2
Fig.1 Synthesis of dithioaryl (TCEP) pyridazinedione and the incubation with Fab.1,2
Equipped with extensive experience in the use of maleimides and other technologies for conjugation to cysteines, Creative Biolabs offers comprehensive Fab-based FDC development services using our various rebridging methods. Our services include but not limited to:
- Customized linker design and synthesis
- Homogenous FDCs generation
- In vitro and in vivo evaluation
With advanced disulfide bonds rebridging platform, services from Creative Biolabs will be your best companion in creating customized Fab-based FDCs. Please contact us for more information and a detailed quote.
References
- Lee, Maximillian TW, et al. "Next-generation disulfide stapling: reduction and functional re-bridging all in one." Chemical Science 7.1 (2016): 799-802.
- Distributed under Open Access License CC BY 4.0, without modification.
For Research Use Only. NOT FOR CLINICAL USE.

Online Inquiry
Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
