Exosome Manufacturing Services
Exosome manufacturing is aimed at scaling up manufacturing yields and controlling purity, increasing manufacturing efficiency and output to meet high-volume exosome needs. Creative Biolabs offers solutions for large-scale extraction and purification of exosomes, suitable for the requirements of industrial exosome manufacturing. Our services not only support scalable upstream manufacturing of exosomes, but also take into account the characterization, profiling and downstream potential exploration of exosome products to provide customized functional exosomes.
Types of Manufactured Exosomes
|
Animal-derived Exosomes
|
Body fluid-derived Exosomes
|
|
Animal-derived exosomes are derived from animal cells, carrying biomolecules from their cells of origin and can influence other cells in the body. Animal-derived exosomes are being investigated in pharmaceutical research, where they might be used to deliver drugs directly to target cells. Additionally, since these exosomes can cross the blood-brain barrier, they may also potentially be used in treating neurological diseases. They are also being used in regenerative medicine, given their ability to stimulate tissue and organ regeneration, and as potential biomarkers in both animal and human diagnostic applications.
|
Exosomes are tiny, membrane-bound vesicles that are found in various body fluids such as blood, urine, saliva and breast milk. Body fluid-derived exosomes serve as valuable resources for the diagnosis and prognosis of different diseases, particularly cancer. They can provide crucial insight into the molecular mechanisms of diseases and may serve as potential therapeutic targets in the future.
|
|
Plant-derived Exosomes
|
Microbial-derived Exosomes
|
|
A series of nano-vesicles or exosome-like nanoparticles in a specified size range can be prepared from a variety of plant samples, including ginger root, rose, ginseng, and grape, which inherit the functional components of the donor plant and have medicinal potential.
|
Membranous vesicles secreted by microorganisms are involved in processes such as microbial community communication, host infection and survival defense. The preparation and study of microbial exosomes facilitate the understanding of their diversity and microbial community behavior.
|
Quick Customization of Your Desired Exosomes
Request Your Customized Exosomes Price
Exosome Manufacturing Process
|
1 Cell line culture and expansion or exosome donor preparation
|
Several rounds of division and expansion to generate a large number of exosome-producing cells or selection of suitable exosome donors from other sources, such as plants, microorganisms and milk, depending on the expected requirements.
|
|
2 Exosome Manufacturing
|
Appropriate exosome manufacturing methods and necessary modification strategies are developed based on the starting amount of exosome donor material and the intended downstream application. Our manufacturing methods include centrifugation, tangential flow filtration, size exclusion, and affinity column chromatography. In addition, Creative Biolabs offers a wide range of services for engineering exosomes including labeling, targeted modification, cargo loading, and more.
|
|
3 Exosome verification
|
Comprehensive characterization and profiling services to verify exosome products, including characterization identification of particle size, morphology and markers and multi-omics profiling of exosome fractions.
|
|
4 Lyophilization of exosomes
|
We can also take freeze-drying measures to obtain exosome powder for easy transportation and storage.
|
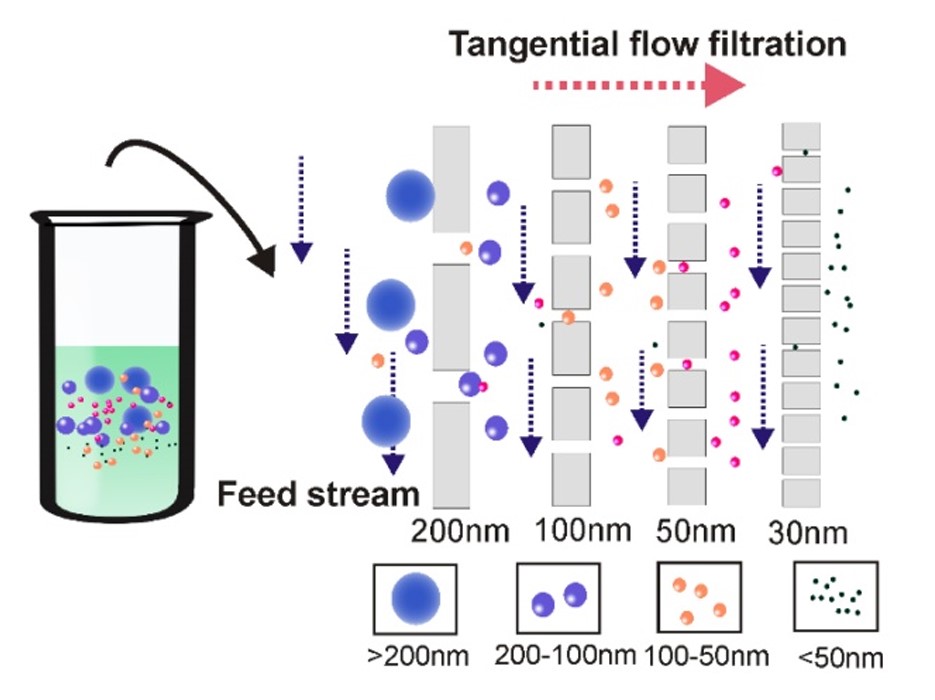 Fig. 1 Tangential flow filtration ensures highly efficient ultrafiltration.1
Fig. 1 Tangential flow filtration ensures highly efficient ultrafiltration.1
Evaluation Services for Manufactured Exosomes
Multiple exosome manufacturing process attributes including efficiency, flexibility, reliability, ease of use and automation are taken into account and collectively affect the yield, purity and physicochemical properties of the obtained exosomes. Therefore, Creative Biolabs is also equipped with a range of inspection services to confirm reliable exosome products that facilitate the subsequent application of exosomes.
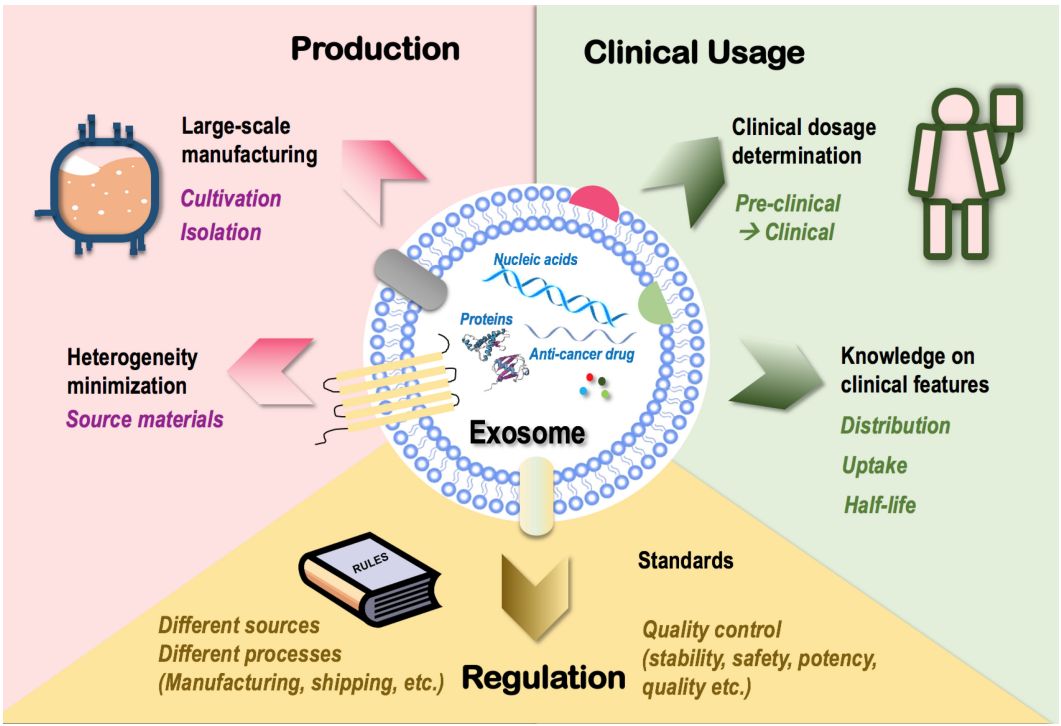 Fig. 2 Challenges in exosome applications.2
Fig. 2 Challenges in exosome applications.2
Advancing the research and application expansion of exosomes requires overcoming the problem of balancing yield and purity in large-scale exosome manufacturing. Creative Biolabs has built a complete exosome manufacturing platform to provide comprehensive services for exosome manufacturing, profiling and quality validation. Please contact us to discuss your needs.
References
-
Yang, Dongbin, et al. "Progress, opportunity, and perspective on exosome isolation-efforts for efficient exosome-based theranostics." Theranostics 10.8 (2020): 3684.
-
Dai, Xiaofeng, Yongju Ye, and Fule He. "Emerging innovations on exosome-based onco-therapeutics." Frontiers in Immunology 13 (2022): 865245.
For Research Use Only. Cannot be used by patients.
Related Services:

 Fig. 1 Tangential flow filtration ensures highly efficient ultrafiltration.1
Fig. 1 Tangential flow filtration ensures highly efficient ultrafiltration.1
 Fig. 2 Challenges in exosome applications.2
Fig. 2 Challenges in exosome applications.2









