Premium Stem Cell-derived Exosome Production Service
Overview Services Features FAQs
Our premium stem cell-derived exosome production services at Creative Biolabs offer a comprehensive solution for clients looking to obtain exosomes derived from various sources of stem cells, including but not limited to human umbilical cord mesenchymal stem cells (HUCMSC) and human adipose-derived mesenchymal stem cells (ADMSC). Our services are designed to cater to the demands for exosomes of the high quality needed for further applications.
Overview
-
Molecule Carriers: They transfer various molecules (proteins, lipids, RNA) to other cells, influencing physiological processes.
-
Regenerative Properties: These exosomes carry the regenerative and therapeutic traits of their parent stem cells.
-
Ethical and Practical Advantages: They avoid the ethical and practical challenges associated with using stem cells directly.
-
Regenerative Medicine Potential: The potential significance of exosomes in tissue renewal and repair is being investigated.
-
Immunomodulation: They have the potential to modulate immune responses effectively.
-
Natural Targeting Ability: They can naturally home in on target cells with minimal immunogenicity.
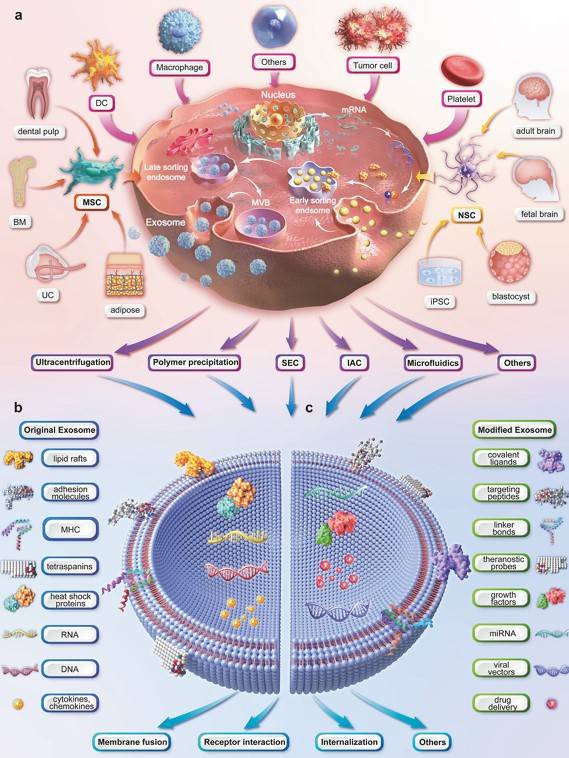 Fig.1 Illustration of the upstream measures of exosome therapy.1, 3
Fig.1 Illustration of the upstream measures of exosome therapy.1, 3
Stem Cell-Derived Exosome Production Services
-
Exosome Sources
-
HUCMSC: Known for their high yield and potent regenerative properties, ideal for therapeutic and regenerative research.
-
ADMSC: Abundant and easily obtainable, these cells offer a robust source for exosome production with a variety of potential applications in disease modeling and regenerative medicine.
-
Other Stem Cell Sources: We can also source and produce exosomes from other stem cell types, tailored to your specific research needs.
|
-
Production Process
-
Stem Cell Culture: We sustain the vitality and functionality of stem cells by cultivating them under optimal circumstances.
-
Exosome Isolation: Separating exosomes using validated methods such as size-exclusion chromatography, filtration, and ultracentrifugation.
-
Characterization: Each batch of exosomes undergoes rigorous characterization to confirm purity, size, and functionality, using methods such as NTA, TEM, and WB.
|
-
Customization Options
-
Custom cell sourcing and culture conditions.
-
Exosome labeling and functionalization.
-
Large-scale production for high-demand projects.
|
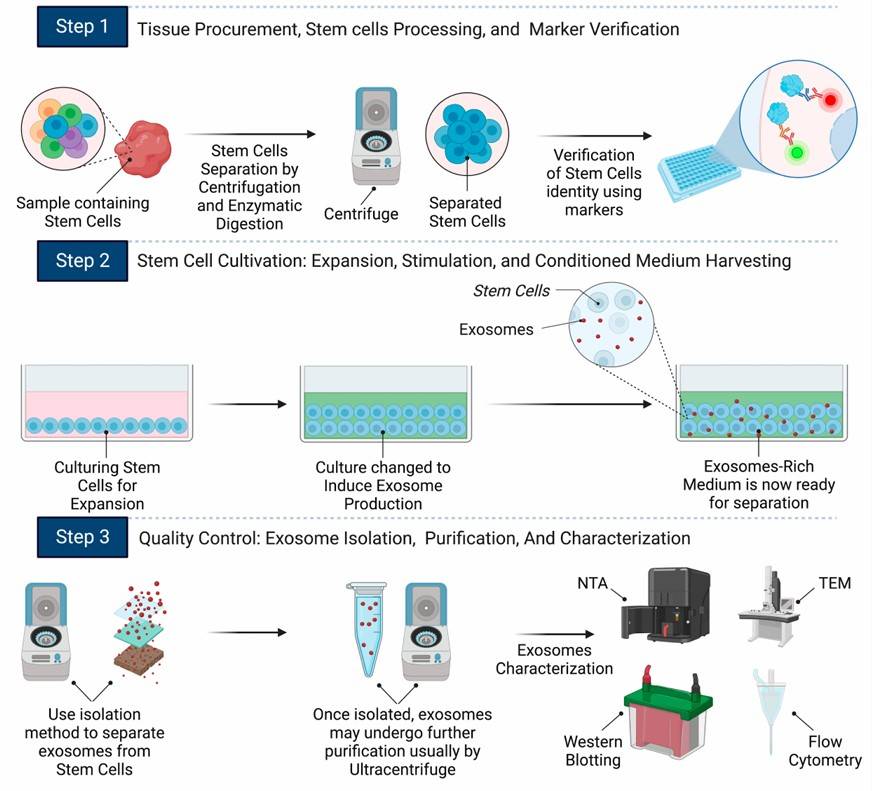 Fig.2 Comprehensive flowchart of exosome production from stem cells.2, 3
Fig.2 Comprehensive flowchart of exosome production from stem cells.2, 3
Features
-
High Quality and Purity: Our exosomes are isolated using state-of-the-art techniques, ensuring minimal contaminants and high functional integrity.
-
Scalability: From small-scale research to large-scale production, our services are designed to scale with your project's needs.
-
Comprehensive Characterization: Each exosome batch is thoroughly characterized, providing you with detailed data on size distribution, concentration, and surface markers.
-
Flexibility and Customization: We offer flexible service options, allowing for customization in cell sourcing, exosome isolation techniques, and delivery formats.
-
Expert Support: Our team of experienced scientists provides full support throughout your project, from initial consultation to final product delivery.
At Creative Biolabs, we combine cutting-edge technology with extensive expertise in exosome biology to deliver reliable and innovative solutions. For more details on our premium stem cell-derived exosome production services, please contact us.
FAQs
Q: How can the exosomes' quality and purity be guaranteed?
A: We employ advanced isolation techniques and thorough characterization methods, such as NTA, and WB, to ensure the highest quality and purity of our exosomes.
Q: How are exosomes produced from stem cells?
A: Exosomes can be isolated from the culture supernatant of stem cells. They are enriched through centrifugation and filtration methods.
Q: What makes your exosome production services unique?
A: Our services stand out due to our advanced production techniques, stringent quality controls, and personalized customer support. We provide adaptable solutions to satisfy certain application requirements and ensure the high purity and reproducibility of exosome preparations.
References
-
Tan, Fei, et al. "Clinical applications of stem cell-derived exosomes." Signal Transduction and Targeted Therapy 9.1 (2024): 17.
-
Abdulmalek, Omar Abdulhakeem Ahmed Yusuf, et al. "Therapeutic Applications of Stem Cell-Derived Exosomes." International Journal of Molecular Sciences 25.6 (2024): 3562.
-
Under Open Access license CC BY 4.0. The image was modified by revising the title.
For Research Use Only. Cannot be used by patients.
Related Services:

 Fig.1 Illustration of the upstream measures of exosome therapy.1, 3
Fig.1 Illustration of the upstream measures of exosome therapy.1, 3
 Fig.2 Comprehensive flowchart of exosome production from stem cells.2, 3
Fig.2 Comprehensive flowchart of exosome production from stem cells.2, 3









