- Home
- UTC Development
- Bispecific ADC Development
- Biparatopic Receptor based Bispecific ADC Development
- Biparatopic CEA-targeted Bispecific ADC Development
Biparatopic CEA-targeted Bispecific ADC Development Service
Antibody-drug conjugates (ADC) are emerging as powerful anticancer treatments and several ADCs have been approved for cancer treatment. ADC is designed to be stable in circulation and release potent cytotoxic drugs in tumor cells upon antigen-specific binding, uptake, and degradation. Efficient internalization and proteolysis in lysosome are essential for a successful ADC. However, for many cell surface proteins and carbohydrate structures on tumor cells, they are insufficient for an effective ADC internalization. Recently, the bispecific antibody (bsAb) approach has been found that can overcome this limitation and have been used in ADCs development.
Creative Biolabs is a leading developer and manufacturer focused on high-quality ADCs design and construction, and we can accelerate your program timelines, mitigate risks in the process, and fast tracke your program to completion. Our state-of-the-art mAb and ADC facility, and expert scientific team can optimize success from innovation through commercialization. Now we provide a full range of bispecific biparatopic ADCs development services targeting CEA antigen.
Background
Introduction of CEA
Carcinoembryonic antigen (CEA) is a glycosyl phosphatidyl inositol (GPI) cell-surface-anchored glycoproteins involved in cell adhesion. The carcinoembryonic antigen family is composed of 29 genes, and 18 of which are normally expressed in humans. CEA serves as functional colon carcinoma L-selectin and E-selectin ligand, which may play a critical role in the metastatic dissemination of colon carcinoma cells. Immunologically, CEA is a member of the CD66 cluster of differentiation (including CD66a-CD66f). Normally, CEA is expressed by columnar and goblet cells in gastrointestinal tissue during fetal development, but the production stops before birth. Thus, CEA is usually present at very low levels in the blood of healthy adults. However, it is overexpressed on some types of cancer cells such as colonic cancer cells, which means that it can be used as a tumor marker in clinical tests. To date, CEA has been regarded as a vital prognostic marker for colorectal cancer diagnosis and therapy. Serum levels of CEA can also be elevated in heavy smokers.
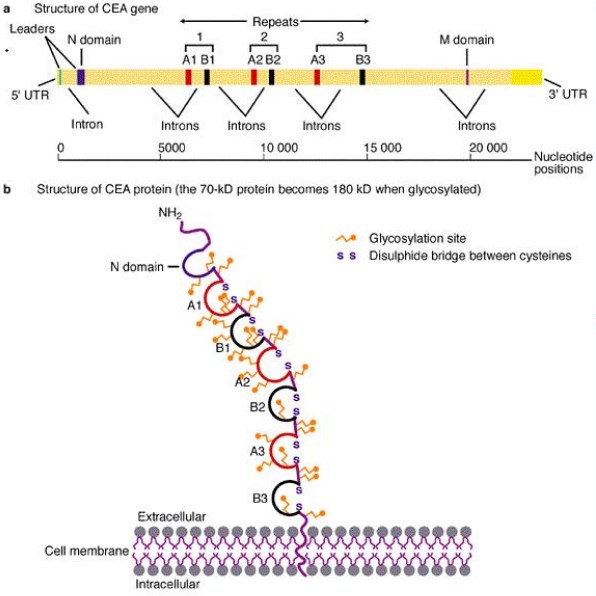 Fig.1 Schematic representation of the human CEA gene and protein.
Fig.1 Schematic representation of the human CEA gene and protein.
Biparatopic CEA-targeted Bispecific Antibody
Bispecific biparatopic antibody presents significant advantages compared with conventional monovalent antibody, including enhanced internalization and trafficking to the lysosomes by simultaneously targeting two different epitopes on the same antigen with a bispecific entity. Besides, it has been used in novel ADC development and showed enhanced internalization and trafficking to the lysosome by inducing clustering and cross-linking of receptors. Therefore, bispecific biparatopic antibody could be a new and effective component used in more potent ADCs construction.
A biparatopic antibody (BpAb) targeting CEA has been developed that has the capability of binding 2 different non-overlapping epitopes on the same target antigen molecule CEA. The biparatopic antibody demonstrated the advantage of biparatopic binding over conventional F(ab')2 binding. Besides, in vivo study results in nude mice xenografted with the human colon carcinoma T380 showed that the BpAb has higher tumor uptake than that of its parental F(ab')2. Thus, the biparatopic antibody (BpAb) may be used for ADC development to achieve a more effective internalization of ADC and more powerful tumor cell killing.
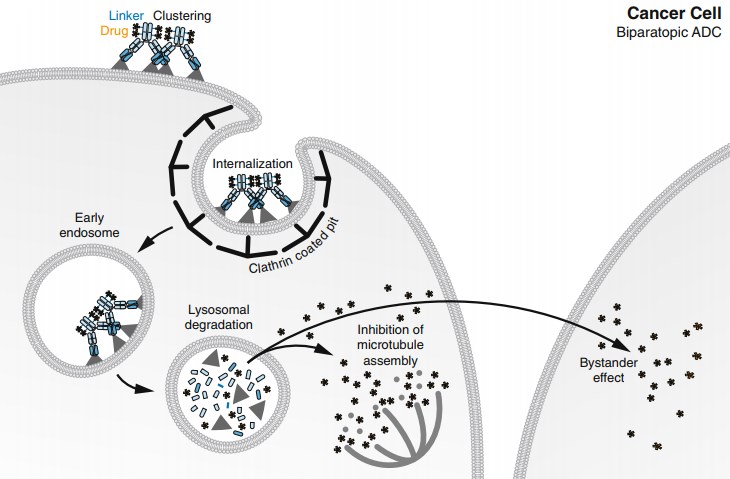 Fig.2 Mode of action of biparatopic ADC.1
Fig.2 Mode of action of biparatopic ADC.1
Our Service
Biparatopic CEA-targeted Bispecific ADCs Services
Creative Biolabs is an acknowledged supplier in ADCs development services. We are committed to offering complete ADCs development and characterization services for more than ten years. Besides, we also provide various ADCs related products such as specific antibodies, potent drugs, and linker-payloads. We are confident that our services and products will promote your innovative bispecific biparatopic ADCs development against CEA protein. Please contact us for more information if you are interested in our services.
Our ADCs development services including:
- ADC Antibody Screening
- DrugLnk™ Custom Synthesis
- Antibody Design and Conjugation
- ADC in vitro Analysis
- ADC in vivo Analysis
Highlights
- Enhanced Internalization Efficiency: Biparatopic CEA-targeted bispecific ADCs offer enhanced internalization by binding two distinct epitopes on the CEA antigen, promoting superior uptake and degradation within tumor cells, which is essential for maximizing ADC efficacy in cancer treatment.
- Bispecific Biparatopic Design: By utilizing a bispecific biparatopic antibody approach, Creative Biolabs' ADCs services ensure more efficient targeting of CEA-expressing tumor cells, enabling more effective drug delivery and improved therapeutic outcomes.
- Superior ADC Construction: The integration of bispecific biparatopic antibodies into ADC development significantly enhances the internalization and lysosomal trafficking of ADCs, ultimately improving their therapeutic potential against CEA-overexpressing tumor cells.
- Comprehensive ADC Development: Creative Biolabs provides complete ADC development services, from antibody design and drug-linker synthesis to in vitro and in vivo analysis, tailored for bispecific biparatopic ADCs targeting the CEA antigen, accelerating your program towards clinical success.
- Cutting-Edge Facility and Expertise: With a state-of-the-art facility and a team of expert scientists, Creative Biolabs ensures seamless biparatopic ADC development, from innovation through to commercialization, mitigating risks and enhancing timelines.
FAQ
-
Q: What are bispecific biparatopic CEA-targeted antibody-drug conjugates (ADCs)?
A: Bispecific biparatopic ADCs targeting carcinoembryonic antigen (CEA) are designed to bind two distinct epitopes on the same antigen, enhancing internalization into tumor cells. This approach increases the efficiency of drug delivery, potentially improving the cytotoxic effects on cancer cells, especially in colorectal cancer treatment.
-
Q: How does Creative Biolabs support the development of biparatopic CEA-targeted ADCs?
A: Creative Biolabs provides comprehensive ADC development services, including antibody screening, conjugation, and in vitro/in vivo analysis. Our expertise in biparatopic ADCs, particularly targeting CEA, helps enhance internalization and efficacy, ensuring faster program completion and optimized therapeutic outcomes.
-
Q: What is the role of CEA in ADC targeting?
A: Carcinoembryonic antigen (CEA) is overexpressed in several cancers, including colorectal cancer, making it a valuable target for ADCs. Biparatopic antibodies designed to target CEA can improve internalization and drug delivery, potentially leading to enhanced tumor cell destruction.
-
Q: Why are bispecific antibodies beneficial for ADC internalization?
A: Bispecific antibodies, particularly biparatopic ones, enhance ADC internalization by binding to two distinct epitopes on the same antigen, such as CEA. This dual engagement promotes receptor clustering, leading to more efficient internalization into the tumor cells and improved therapeutic efficacy.
-
Q: What makes CEA a suitable target for bispecific biparatopic ADCs?
A: CEA is highly expressed in certain cancers, such as colorectal cancer, but present at low levels in normal tissues, making it an ideal target for ADCs. Bispecific biparatopic antibodies can improve the internalization of ADCs by targeting two distinct epitopes on CEA, enhancing therapeutic efficacy.
Published Data
The CEA/CD3 bispecific antibody developed in this study targets carcinoembryonic antigen (CEA) on tumor cells and CD3 on T cells, enabling T cells to specifically attack CEA-expressing cancer cells, such as those in colorectal cancer. This antibody was engineered to reduce the risk of cytokine release syndrome (CRS), a common side effect of T cell engagers, by modifying the CD3 binding to be monovalent and affinity-reduced. Two variants were tested, and CEA/CD3-v2 was selected as the lead candidate due to its potent cytotoxicity against CEA-positive cancer cells and significantly reduced cytokine release. In both in vitro and in vivo experiments, CEA/CD3-v2 demonstrated strong antitumor efficacy, particularly in combination with the PD-L1 inhibitor atezolizumab. This combination treatment showed enhanced tumor growth inhibition and increased infiltration of tumor-killing T cells, suggesting that CEA/CD3-v2 could be a promising therapeutic for CEA-positive cancers.
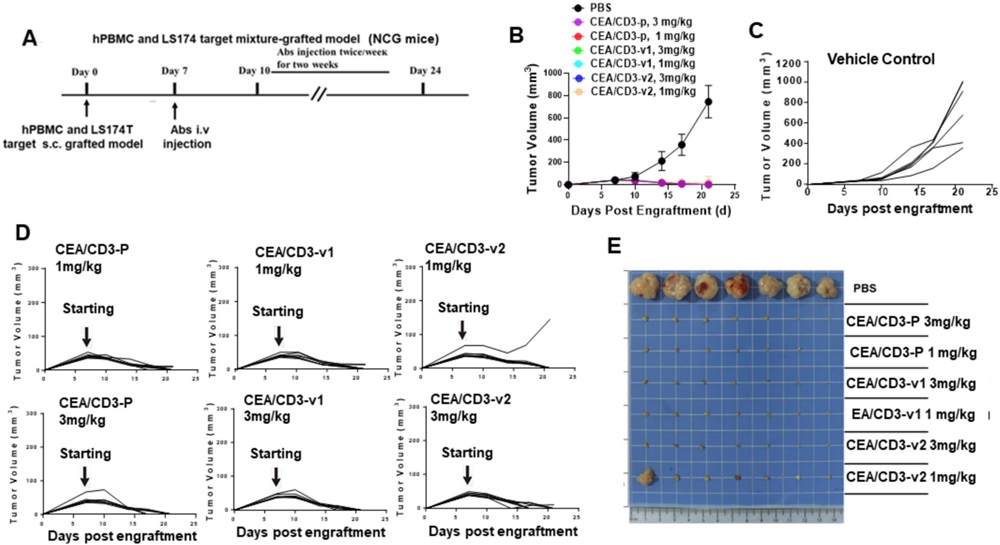 Fig.2 In vivo efficacy results of CEA/CD3 bsAb in animal models.2
Fig.2 In vivo efficacy results of CEA/CD3 bsAb in animal models.2
Featured Products
Anti-CEA ADC
| Catalog | Product Name | Antibody |
| ADC-W-381 | Anti-CEACAM1 (clone T84.66) scFv-dextran T-40-MMC ADC | Anti-CEACAM1 Antibody, clone # anti-CEA scFv T84.66 |
| ADC-W-472 | Anti-CEACAM5-Phy-lys-SN-38 ADC | Humanized Anti-CEACAM5 lgG1 Antibody |
| ADC-W-974 | Anti-CEACAM6 (Sulesomab)-SMCC-DM1 ADC | Anti-CEACAM6 IgG1-Fab' fragment, Sulesomab |
| ADC-W-2204 | Anti-CEACAM8 (Besilesomab)-SMCC-DM1 ADC | Humanized Anti-CEACAM8 IgG1-kappa antibody, Besilesomab |
| ADC-W-2579 | Anti-CEACAM5-MC-MMAF ADC | Humanized Anti-CEACAM5 IgG antibody |
References
- Chalouni, C.; Doll, S. Fate of antibody-drug conjugates in cancer cells. Journal of Experimental & Clinical Cancer Research. 2018, 37(1): 20.
- Wang, Ninghai, et al. "An optimal antitumor response by a novel CEA/CD3 bispecific antibody for colorectal cancers." Antibody Therapeutics 4.2 (2021): 90-100.
For Research Use Only. NOT FOR CLINICAL USE.

Online Inquiry
Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
