Backgrounds Published Data Our Services
Creative Biolabs boasts unparalleled expertise in antibody development and the synthesis of recombinant proteins. Our comprehensive suite of services for bispecific antibodies (BsAb) encompasses the entire spectrum, from design and engineering to manufacturing and the rigorous analysis of BsAbs tailored for immunological applications.
Overview
The human immune system plays a pivotal role in safeguarding overall health by shielding the body against a multitude of foreign intrusions and the irregular behavior of self-cells. While the immune system is indispensable for maintaining human health, there are instances when aberrant or excessive immune responses lead to tissue damage, organ growth abnormalities, and altered organ function. A spectrum of immunological disorders, including chronic inflammation, autoimmune diseases, hypersensitivity, immune deficiencies, and transplant rejection, underscores the intricate nature of the immune system's operation.
BsAbs are engineered to concurrently target two distinct antigens or epitopes, uniting the specificities of two antibodies. BsAbs endowed with this "two-target" functionality can effectively interfere with multiple surface receptors or ligands linked, for example, to cancer, proliferation, or inflammatory processes. Consequently, BsAbs possess the capability to disrupt receptor signaling, making them invaluable tools in the field of immunology.
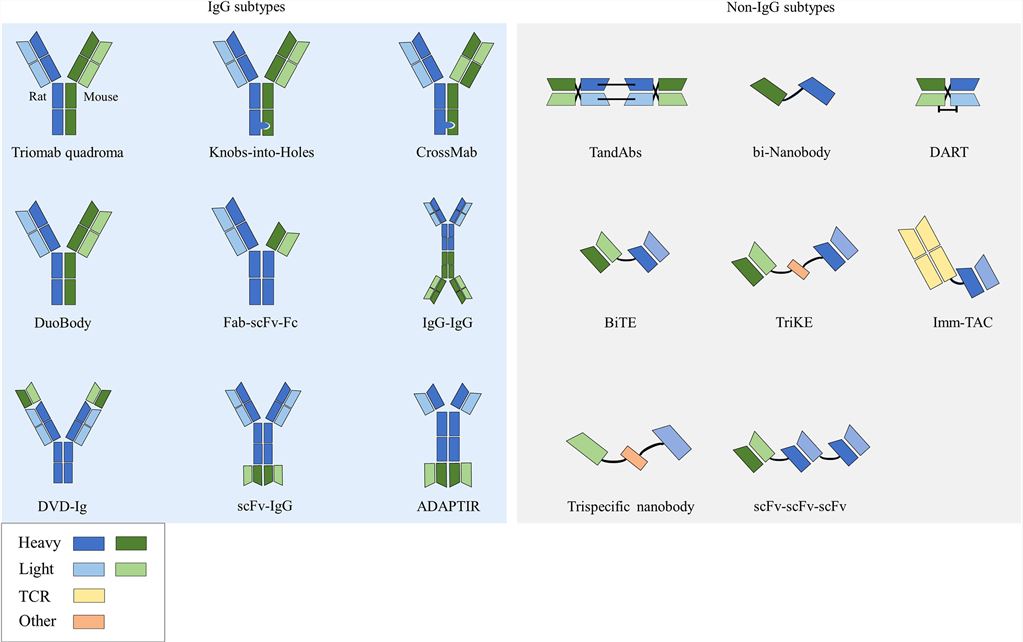
Fig.1 The structure of BsAbs.1
BsAb Interfering Receptor Signaling
Signaling networks exert dominion over a myriad of intracellular and intercellular biological functions and behaviors, orchestrating processes ranging from regulated catabolism and metabolism to the intricate choreography of programmed cell proliferation or apoptosis. In numerous instances, the judicious supplementation of deficient signaling factors or the targeted blockade of aberrant signaling pathways can offer a means to control symptoms and mitigate disease progression. In stark contrast to their monospecific antibody counterparts, BsAbs possess the remarkable ability to concurrently disrupt the activities of two or more receptors or ligands within one or across two signaling pathways. This unique attribute of BsAbs curtails the likelihood of escape mechanisms, amplifying the efficacy of therapeutic interventions. Excessive or dysfunctional immune responses, such as self-targeting inflammatory reactions, underpin a multitude of disorders and autoimmune diseases. Prominent examples include rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, Behcet's disease, psoriasis, inflammatory bowel disease, allergies, and cardiovascular disorders.
Published Data
|
Data 1
|
ABL001, a Bispecific Antibody Targeting VEGF and DLL4, with Chemotherapy, Synergistically Inhibits Tumor Progression in Xenograft Models
|
|
Journal
|
International Journal of Molecular Sciences
|
|
Published
|
2020
|
|
Abstract
|
Delta-like-ligand 4 (DLL4) emerges as a promising target with the potential to enhance the efficacy of vascular endothelial growth factor (VEGF) inhibitors. Simultaneously blocking the VEGF/VEGFR and DLL4/Notch signaling pathways induces more robust anti-cancer effects through synergistic anti-angiogenic mechanisms in xenograft models. A bispecific antibody designed to target both VEGF and DLL4 exhibits greater in vitro and in vivo biological activity when contrasted with monoclonal antibodies targeting VEGF or DLL4 alone. Currently, it is undergoing evaluation in a phase 1 clinical trial involving cancer patients who have been pre-treated with either intensive chemotherapy or targeted therapy. Nonetheless, the combined effects of BsAb and chemotherapy on tumor vasculature and tumor progression remain undisclosed. Consequently, the study sought to investigate the impact of BsAb, both in isolation and in combination with paclitaxel and irinotecan, in human gastric and colon cancer xenograft models. The combination therapy exhibited a synergistic inhibition of tumor progression, surpassing the effects of each monotherapy in isolation. Tumors subjected to this combination therapy displayed marked tumor vessel regression and the induction of apoptotic tumor cells, which is indicative of potential tumor vessel normalization. These results collectively suggest that the combination therapy involving ABL001 and either paclitaxel or irinotecan represents a more promising clinical strategy for the treatment of cancer patients.
|
|
Result
|
This investigation has yielded a BsAb aimed at the dual targeting of VEGF and DLL4, representing a novel anti-angiogenic cancer therapeutic. The BsAb not only amplifies the impact of VEGF inhibitors but also holds the potential to surmount resistance encountered in anti-VEGF therapy.
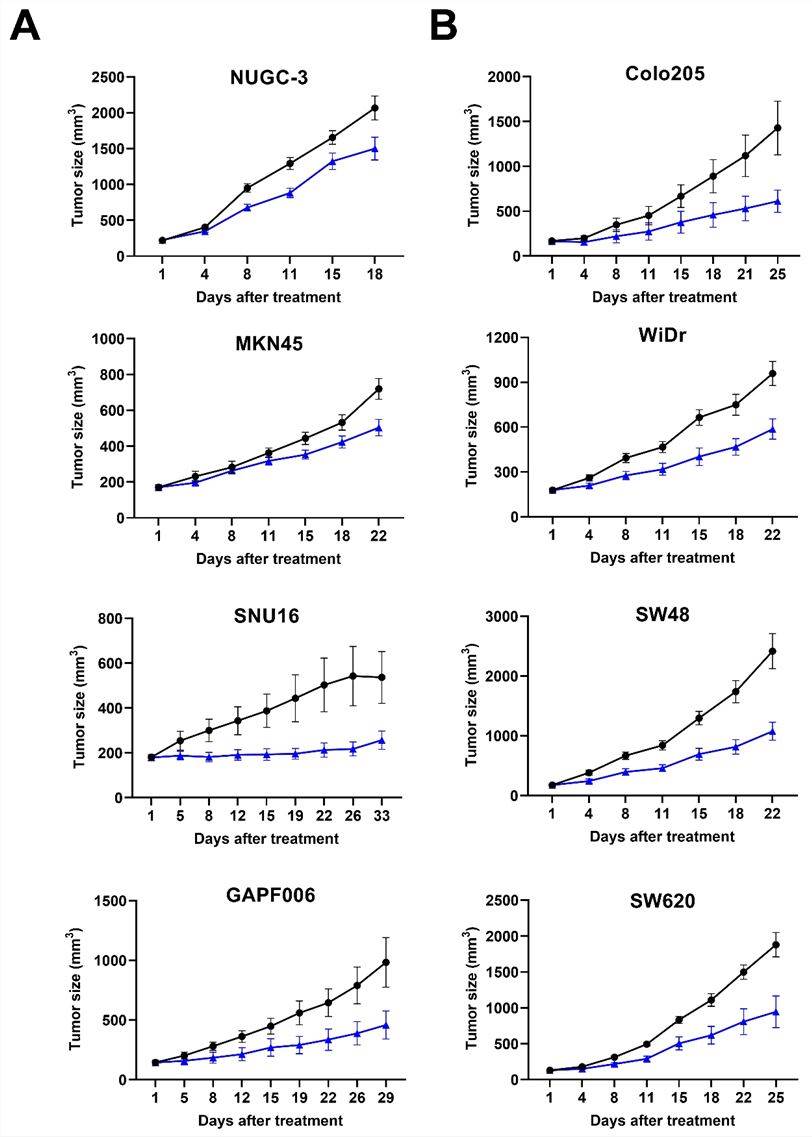
Fig.2 The BsAb exerted potent inhibition of tumor progression across diverse xenograft models of human gastric and colon cancers.2
|
Our Services
Creative Biolabs has superior BsAb production platforms and a highly accomplished research team, assuring our clientele of the provision of high-quality BsAb. We offer a diverse range of immunology products, including minibody designed for various functional antigens, capable of serving as dual inhibitors or immune cell engagers. Additionally, our portfolio includes the Fab-IgG BsAb generation service. This translates into a dual advantage for our customers, facilitating both time and budget savings while availing themselves of our BsAb services. Please contact us for more information.
References
1. Kang, Jingyue, Tonglin Sun, and Yan Zhang. "Immunotherapeutic progress and application of bispecific antibody in cancer." Frontiers in Immunology 13 (2022): 1020003. Distributed under open access license CC BY 4.0, without modification.
2. Yeom, Dong-Hoon, et al. "ABL001, a bispecific antibody targeting VEGF and DLL4, with chemotherapy, synergistically inhibits tumor progression in xenograft models." International Journal of Molecular Sciences 22.1 (2020): 241. Distributed under open access license CC BY 4.0, without modification.
Our products and services are for research use only, and not for use in diagnostic or therapeutic procedures.
Welcome! For price inquiries, we will get back to you as soon as possible.
To order, please email
INQUIRY






