Creative Biolabs is specialized in conducting pharmacokinetic and pharmacodynamic (PK/PD) data analysis for antibody drug discovery, preclinical, and clinical studies. We offer either model dependent or independent analysis services to evaluate the PK/PD of your therapeutic bispecific antibodies (BsAbs). Our analysis information will provide you with valuable insights into the design of preclinical and clinical studies of your BsAbs.
Antibody drugs are expected to display a variety of desirable characteristics, such as great solubility, stability, high selectivity and specificity, long persistence in the body, and low risk for bioconversion to toxic metabolites. Antibodies have been studied in a number of disease conditions, with effects generated via a complex array of mechanisms. One possible reason for the steady success of the therapeutic antibody development is the application of the PK/PD concepts at all stages of the drug development.
Population pharmacokinetics is one of the PK/PD concepts in drug development. It allows to quantify the typical disposition characteristics and sources of pharmacokinetic variability within the studied populations. Meanwhile, it is aimed at identification and quantification of the impact of covariates on systemic drug exposure, and measurement of their potential implications for clinical dosing. PK/PD analysis serves as an important component of the drug discovery and development process. Usually, antibody drugs display much more complex PK/PD properties than those common small-molecule drugs.
Antibody Absorption
Due to the limited stability in gastrointestinal tract and poor permeability through the gut wall, BsAbs have negligible oral bioavailability. Thus, BsAbs are usually administered by parenteral routes, including subcutaneous (s.c.), intravenous (i.v.), and intramuscular (i.m.) administrations. Direct delivery to the site of action has also been used to attain high local concentrations, such as intraperitoneal (i.p.) and intrapleural (i.pl.) administrations.
Antibody Distribution
The distribution of BsAbs is measured by the rate of extravasation in tissue, the rate of distribution within tissue, the rate and extent of antibody binding in tissue, as well as the rate of elimination from tissue. For BsAbs, diffusion across vascular endothelial cells is extremely slow, and convection is considered to be the major mechanism responsible for the transport of antibody from blood fluid to interstitial fluids of tissue. Moreover, the rate of extravasation by convective transport, or the movement of antibody into tissue by “solvent drag,” is affected by the rates of fluid movement from blood to tissue, as well as the sieving effect of paracellular pores in the vascular endothelium. In addition, the rate of antibody elimination from tissue can be primarily measured by the convective elimination clearance and by the rate of antibody catabolism within tissue.
Antibody Metabolism and Elimination
Metabolism of BsAb takes place in a range of body tissues and in plasma. With pharmacokinetic modelling, the contribution skin, muscle, liver and gut tissue to the elimination of BsAb was estimated to be 33%, 24%, 16%, and 12%, respectively. Generally, the mechanisms of drug elimination include filtration, secretion, and biotransformation. Renal elimination is a major pathway for small-molecule drug clearance; however, it is dispensable for BsAb, due to its large size that prevents efficient filtration via the glomerulus. Most BsAb elimination takes place via intracellular catabolism, following fluid-phase or receptor-mediated endocytosis.
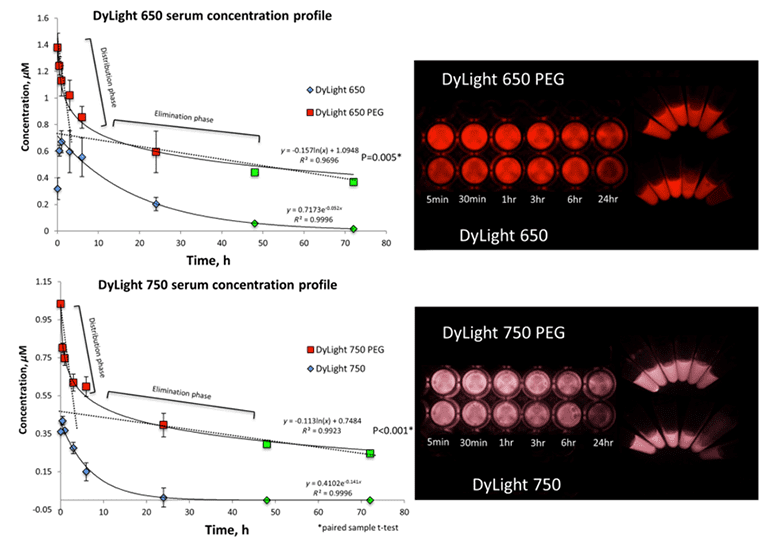 Fig 1. Elimination profiles of PEGylated and non-PEGylated dye-mAb complexes (Maawy, A. A., 2014).
Fig 1. Elimination profiles of PEGylated and non-PEGylated dye-mAb complexes (Maawy, A. A., 2014).
Creative Biolabs has years' experience in antibody discovery and development, and will offer you the best advice and support to help advance your pharmacokinetics programs. We can design proper pharmacokinetic studies and accomplish pharmacokinetic calculations. Our high-quality PK/PD analysis services will greatly contribute to the successes of your projects. We also provide various other services regarding BsAb development. Please feel free to contact us for more information and a detailed quote.
Reference
-
Maawy, Ali A., et al. "Specific tumor labeling enhanced by polyethylene glycol linkage of near infrared dyes conjugated to a chimeric anti-carcinoembryonic antigen antibody in a nude mouse model of human pancreatic cancer." Journal of Biomedical Optics 19.10 (2014): 101504-101504. https://doi.org/10.1117/1.JBO.19.10.101504. (Under open access license CC BY 4.0, without modification.)
Our products and services are for research use only, and not for use in diagnostic or therapeutic procedures.
Welcome! For price inquiries, we will get back to you as soon as possible.
To order, please email
INQUIRY


 Fig 1. Elimination profiles of PEGylated and non-PEGylated dye-mAb complexes (Maawy, A. A., 2014).
Fig 1. Elimination profiles of PEGylated and non-PEGylated dye-mAb complexes (Maawy, A. A., 2014).


