scFv1-PEG-scFv2 is a bispecific antibody (BsAb) composed of two single chain variable fragments (scFvs) and a cross-linker containing polyethylene glycol (PEG), which can simultaneously recognize two different antigens and introduce other functional molecules through the cross-linker, such as fluorophores, peptides, siRNA, toxic drugs, etc., to increase its multifunctionality and therapeutic effect. scFv is the smallest unit of an antibody molecule that can bind antigen. It consists of a variable heavy chain (VH) and a variable light chain (VL) connected by a flexible linker peptide. PEG is a polymer widely used in the biomedical field. It has good biocompatibility, water solubility and flexibility and can improve the pharmacokinetic properties of scFv, extend its half-life in vivo, and reduce its immunogenicity. Compared with other BsAb formats, scFv1-PEG-scFv2 has higher flexibility and diversity, as they can introduce different types and numbers of functional molecules through the cross-linker to achieve multiple targeting or multiple effects. However, scFv1-PEG-scFv2 also has some limitations, such as the selection of cross-linker, reaction conditions, product purification, etc., which will affect the production efficiency and quality of scFv1-PEG-scFv2.
Structural Features of scFv1-PEG-scFv2
scFv1-PEG-scFv2 is a BsAb format that consists of two scFvs and a cross-linker containing PEG. The scFvs are the antigen-binding domains of an antibody, which are composed of VH and VL domains. The VH and VL domains are connected by a flexible linker peptide, which is usually 15-20 amino acids long and contains glycine and serine residues with dispersed hydrophilic residues for increased solubility. The linker peptide keeps the C-terminus of one variable domain and the N-terminus of the other domain at a distance that favors proper folding and formation of the antigen-binding site while also minimizing oligomerization of the scFv. The scFvs can be arranged in either VL-linker-VH or VH-linker-VL configurations, and both configurations can generate functional scFvs, but some specific scFvs may perform better in one configuration than the other. The cross-linker containing PEG is used to conjugate the two scFvs and introduce additional functionalities. PEG is a polymer that has good biocompatibility, water solubility and flexibility, and can improve the pharmacokinetic properties of scFv, such as extending its half-life in vivo and reducing its immunogenicity. The cross-linker can also contain other functional molecules, such as antibody fragments, peptides, siRNA, toxic drugs, or fluorophores, which can enhance the multifunctionality and therapeutic effect of scFv1-PEG-scFv2. The cross-linker can have different types and lengths, depending on the desired properties and applications of scFv1-PEG-scFv2. The cross-linker reacts with specific sites on the scFvs, forming stable covalent bonds and generating a homogeneous product.
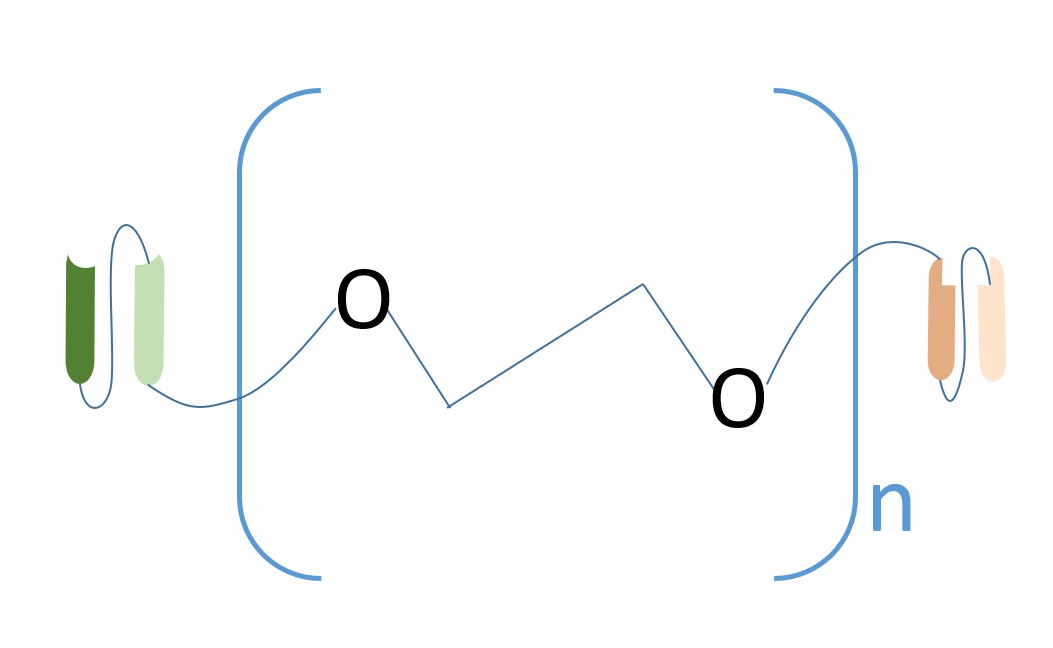
Fig.1 The schematic diagram of the structure of scFv1-PEG-scFv2 (Creative Biolabs)
Generation Methods of scFv1-PEG-scFv2
scFv1-PEG-scFv2 can be generated by two main methods: chemical conjugation and genetic engineering. Chemical conjugation is the process of using a cross-linker with multiple reactive groups to link two scFvs at specific sites, forming stable covalent bonds. The advantage of this method is that it can flexibly choose different types and lengths of cross-linkers, as well as different types and numbers of functional molecules, to achieve multiple targeting or multiple effects. Genetic engineering is the process of using recombinant DNA technology to connect two scFv genes with a linker peptide gene, constructing a fusion protein gene, and expressing the fusion protein in an appropriate expression system. The advantage of this method is that it can efficiently express scFv1-PEG-scFv2 in bacteria, reducing cost and complexity.
Clinical Data of scFv1-PEG-scFv2
scFv1-PEG-scFv2 is a BsAb format that has potential applications in various diseases, especially in cancer therapy. There are no approved scFv1-PEG-scFv2 products yet, possibly because this format is still relatively novel and requires more research and validation. However, there are some scFv1-PEG-scFv2 products in clinical trials, mainly for cancer treatment, such as lymphoma, leukemia, lung cancer, etc. These products are mainly developed by some biotechnology companies or research institutions, such as Creative Biolabs, Ablynx, Roche, etc.
Table 1. scFv1-PEG-scFv2 products in clinical trials
|
Product name
|
Targets
|
Clinical phase
|
Indications
|
Country/region
|
|
ALX-0061
|
IL-6R and albumin
|
Phase II/III
|
Rheumatoid arthritis, systemic lupus erythematosus
|
Europe, USA, Japan
|
|
RG6026
|
CD20 and CD3
|
Phase I/II
|
B-cell malignancies, chronic lymphocytic leukemia, non-Hodgkin lymphoma
|
USA, Europe, Australia
|
|
R0950
|
EGFR and CD3
|
Phase I/II
|
Non-small cell lung cancer, head and neck cancer, colorectal cancer
|
USA, Europe
|
References
1. Ellerman D, et al. Generation of bispecific antibodies by chemical conjugation. In: Kontermann RE, editor. Bispecific Antibodies. Berlin: Springer; 2011. p. 47-65.
2. Spiess C, et al. Alternative molecular formats and therapeutic applications for bispecific antibodies. Mol Immunol. 2015 Oct;67(2 Pt A):95-106.
3. Kontermann RE, et al. Bispecific antibodies. Drug Discov Today. 2015 Jun;20(7):838-47.
4. Brinkmann U, et al. The making of bispecific antibodies. MAbs. 2017 Jan;9(1):182-212.
5. Holliger P, et al. Engineered antibody fragments and the rise of single domains. Nat Biotechnol. 2005 Sep;23(9):1126-36.
6. Hu S, et al. Minibody: A novel engineered anti-carcinoembryonic antigen antibody fragment (single-chain Fv-CH3) which exhibits rapid, high-level targeting of xenografts. Cancer Res. 1996 Jul 1;56(13):3055-61.
7. Adams GP, et al. Monoclonal antibody therapy of cancer. Nat Biotechnol. 2005 Sep;23(9):1147-57.
8. Verma S, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012 Nov 8;367(19):1783-91.
9. Carter PJ, et al. Next generation antibody drugs: pursuit of the 'high-hanging fruit'. Nat Rev Drug Discov. 2018 Jan;17(3):197-223.
Our products and services are for research use only, and not for use in diagnostic or therapeutic procedures.
Welcome! For price inquiries, we will get back to you as soon as possible.
To order, please email
INQUIRY