Extracellular Vesicle Characterization and Quantification Service
Overview Services Features FAQs
Creative Biolabs offers a comprehensive EV characterization and quantification service to support the understanding of the properties and downstream applications of EVs.
Overview
In addition to providing insight into the biogenesis and cargo sorting of EVs, the measurement and characterization of EVs are useful in identifying physiological and pathological indicators. Our Characterization and Quantification Services for Extracellular Vesicles (EVs) are designed to provide comprehensive analysis and measurement solutions for researchers studying EVs. We offer validated technologies and expertise to ensure accurate characterization and quantification of EVs, aiding in advancing your research in this rapidly evolving field.
Services
Since no single method can accurately phenotype, size and enumerate all EVs, thus providing all the necessary information to truly understand the biology of EVs, Creative Biolabs offers a combination of optional methods for the characterization and quantification of EVs, including but not limited to.
-
Nanoparticle Tracking Analysis (NTA): The technique has been recognized by the EVs research field as one of the characterization tools. It is also suitable for the characterization of samples such as liposomes, proteins, and biological nanomaterials.
-
Transmission electron microscopy (TEM): After negative staining of EVs with uranyl acetate and cellulose, the surface features of EVs can be observed by TEM. In the images, these EVs can be clearly distinguished into different sizes.
-
Fluorescence activated Cell Sorting (FACS): Micron-sized latex beads are used to bind EVs. After then, fluorescent antibodies are used to dye the attached EVs, allowing protein markers to be detected. The EVs are ejected in a single row by the nozzle of the flow chamber under the constraint of the sheath fluid, forming a column of EVs, the latter intersecting perpendicularly with the incident laser beam, and the EVs in the column are excited by the laser to produce fluorescence. The optical system collects signals such as fluorescence, light scattering, light absorption or electrical impedance and feeding back to the computer system for statistical analysis of EVs characterization.
-
Western Blotting: The most common technique used in EV protein assessment to suggest the presence of target proteins associated with EVs. During the procedure, purified EVs are treated with a buffered lysate containing denaturant and protease inhibitors. Following electrophoresis, the protein lysates are transferred to membranes so that certain protein targets can be immunoblotted. The approach can provide useful information about the size of the protein molecule.
-
Enzyme-linked immunosorbent assay (ELISA): Purified EVs or EVs lysates can be applied directly to an adsorbent column pretreated with immobilized capture antibodies. The captured EVs are used for another detection antibody. This improves the specificity of the assay for non-interacting antibodies. The ELISA is more rapid and can be used for high-throughput measurements.
-
Tunable Resistive Pulse Sensing (TRPS): TRPS is available as an alternative to NTA for measuring EV concentration and size distribution.
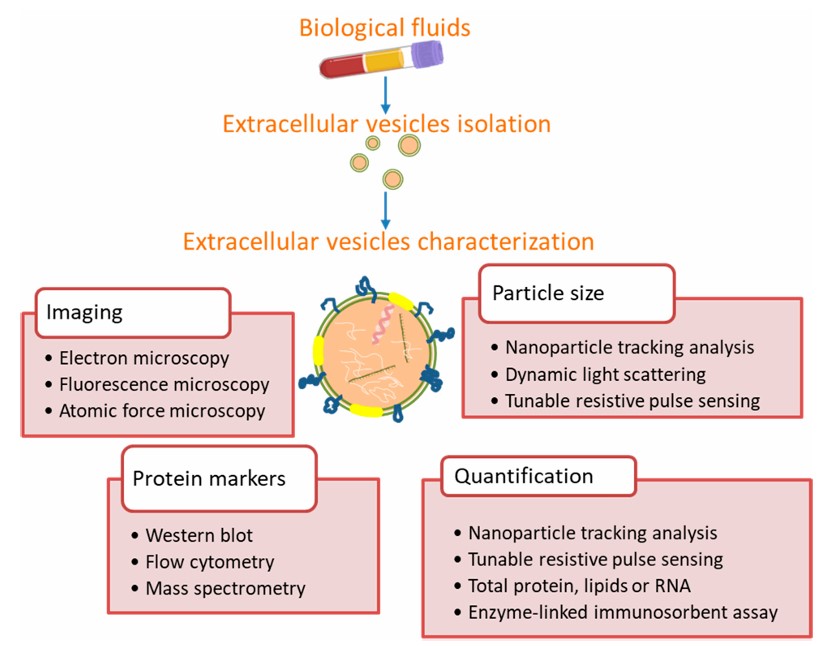 Fig. 1 Methods commonly used for extracellular vesicle characterization from biological fluids.1
Fig. 1 Methods commonly used for extracellular vesicle characterization from biological fluids.1
Features
-
Advanced Technologies: Utilize cutting-edge techniques such as nanoparticle tracking analysis (NTA), flow cytometry, and electron microscopy for precise EV characterization.
-
Customizable Services: Tailor our services to meet your specific research needs, whether it's profiling EV cargo, determining EV size distribution, or assessing EV purity.
-
Expert Consultation: Benefit from the guidance of experienced scientists who specialize in EV research and analysis, ensuring reliable results and interpretation.
-
Comprehensive Reports: Receive detailed reports summarizing EV characteristics, such as concentration, size distribution, morphology, and surface markers.
With our innovative EVs platform, Creative Biolabs provides not only characterization and quantification of EVs, but also in-depth technical support from project planning to data interpretation. Please contact us with your interest.
FAQs
Q: What samples would you need for analysis?
A: You can provide isolated exosomes for us to perform exosome analysis directly, or provide source samples for us to perform exosome isolation and further exosome analysis.
Q: How do you quantify extracellular vesicles?
A: We employ methods such as NTA and BCA to quantify EVs based on particle concentration, size, and protein concentration.
Q: What is the duration required to obtain the outcomes?
A: Turnaround time depends on the scope of analysis and sample complexity. We provide estimated timelines upon project initiation.
Q: Are your services customizable?
A: Absolutely. We work closely with researchers to tailor our services to meet specific research objectives and experimental requirements.
Reference
-
Sandúa, Amaia, Estibaliz Alegre, and Álvaro González. "Exosomes in lung cancer: Actors and heralds of tumor development." Cancers 13.17 (2021): 4330. Under Open Access license CC BY 4.0. The image was modified by revising the title.
For Research Use Only. Cannot be used by patients.
Related Services:

 Fig. 1 Methods commonly used for extracellular vesicle characterization from biological fluids.1
Fig. 1 Methods commonly used for extracellular vesicle characterization from biological fluids.1









