Extracellular Vesicle Manufacturing Service
Overview Services Features FAQs
Overview
The Necessity of Large-Scale Production of Extracellular Vesicles
Extracellular vesicles (EVs) are active vesicles secreted by most cells, and they mediate various physiological and pathological functions, such as cell homeostasis, infection transmission, tumor development, etc., by participating in intercellular material transfer. Thus, the discovery of EVs as mediators of intercellular communication has fueled the exploration of their therapeutic potential. A large number of studies suggest that the drug delivery and therapeutic capabilities of EV can be extended to the treatment of various diseases. Although natural EV or engineered EV are considered to be effective therapeutic drugs, their clinical application remains a challenge. Large-scale and efficient production of EVs with minimal batch-to-batch variability and efficacy variability following compliance with international biologics norms is necessary to enhance the therapeutic potential of EV. Creative Biolabs has established a large-scale mass-production EV platform and continuously optimizes the culture cell process and EV production process, which can provide the most efficient large-scale EV extraction service for global customers.
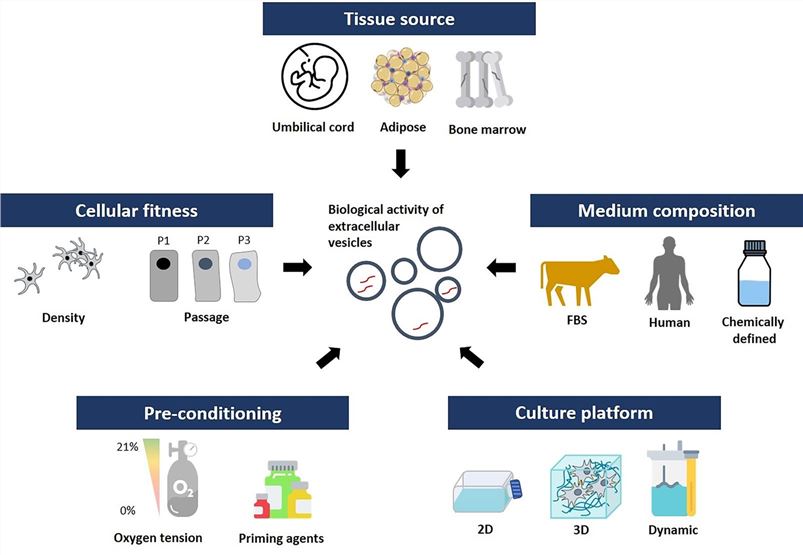 Fig.1 Key considerations for MSC-derived EV production.1,3
Fig.1 Key considerations for MSC-derived EV production.1,3
Services
-
Large-Scale Cell Culture Service
The contents of EV are diverse, depending on their function and the type of donor cell. In addition, the production efficiency and stability of EV are affected by factors such as the microenvironment and culture conditions of the donor cells. Creative Biolabs can provide EV endogenous loading service, endogenous targeting service, and endogenous labeling service by transforming donor cells, strictly control the cell culture conditions, and cultivate the cells specified by customers in high density and on a large scale.
-
Large-scale Extracellular Vesicle Isolation and Purification Service
In previous studies, the most commonly used methods for extracting EV include ultracentrifugation, precipitation, size exclusion chromatography, affinity capture, etc. However, these methods have low yield and high cost, and it is difficult to realize industrialization and clinical application for a large amount of cell supernatant. Therefore, Creative Biolabs has established a large-scale mass-producing EV platform with tangential flow ultrafiltration (TFF) concentration pretreatment combined with classical EV separation methods by introducing advanced technologies and further improving and enhancing them. This method has the advantages of simple operation, controllable parameters, and short time consumption, and can process large volumes of feed liquid, thereby obtaining a large number of high-purity EVs for subsequent use.
-
Extracellular Vesicle Product Testing and Packaging Service
EV products obtained from different batches before and after freeze-thawing need to be tested for mycoplasma, endotoxin, particle size, markers, morphology, drug loading rate, etc., to control the variance of EV products between batches. Then, EV products that meet the standards are freeze-dried into a powder state for long-distance transportation and long-distance storage. Creative Biolabs can provide the most rigorous EV identification service to help customers control batch-to-batch differences and high-quality large-scale freeze-drying technical services to ensure the biological activity of EV.
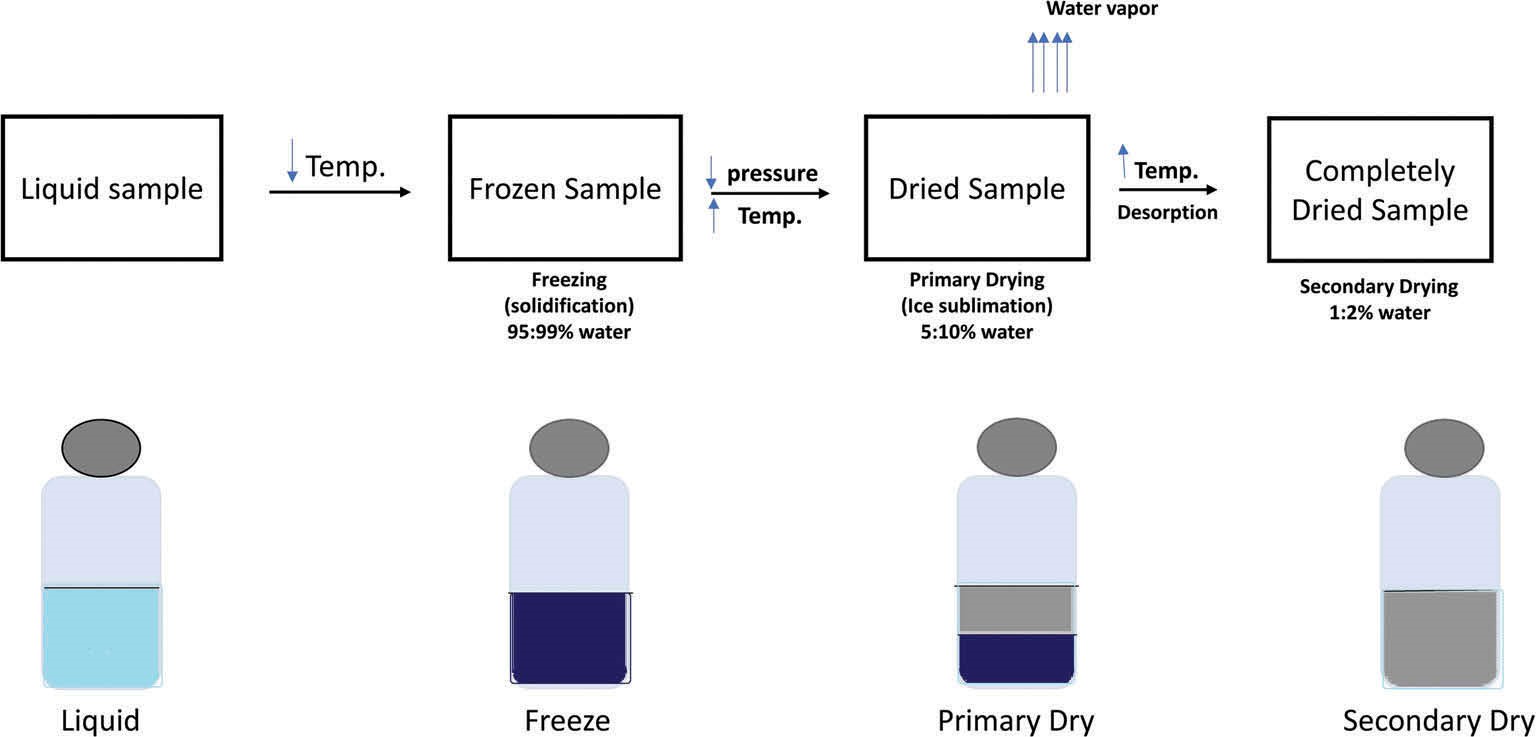 Fig.2 Schematic diagram of freeze drying.2.3
Fig.2 Schematic diagram of freeze drying.2.3
Features
-
Efficient Large-Scale Cell Culture Technology
-
High-Purity Extracellular Vesicle Extraction
-
Comprehensive Quality Inspection and Quality Control
-
Customized Packaging and Storage Services
Creative Biolabs has been continuously innovating EV manufacturing technology to help customers achieve large-scale EV production from research and development to clinical practice. If you need large-scale EVs, please contact us without hesitation to propose your target donor cells and loaded drugs. Our professional team will formulate the most suitable large-scale separation and purification scheme according to the research needs of customers to prepare high-quality EV products of different specifications.
FAQs
Q: What types of cells are your extracellular vesicle extraction services suitable for?
A: Our extracellular vesicle extraction services are suitable for a wide range of cell types, including but not limited to stem cells, tumor cells, and fibroblasts. We offer customized processing based on the client's needs to ensure optimal extraction results.
Q: How do you ensure the purity and quality of the extracellular vesicles?
A: We employ multiple advanced separation techniques, such as ultracentrifugation, size-exclusion chromatography, and tangential flow filtration, along with a comprehensive quality control process, including particle size analysis, electron microscopy observation, and marker identification, to guarantee high purity and quality in each batch of extracellular vesicles.
Q: What is the delivery timeline for your cell culture and extracellular vesicle extraction services?
A: Typically, our delivery timeline depends on the project's scale and complexity. Most projects can be completed within a few weeks, with the exact timeline adjusted according to the client's requirements and our production schedule. We are committed to providing fast and reliable services to our clients, ensuring that their projects are delivered on time.
Q: Do you offer small-scale pilot extraction services?
A: Yes, we provide small-scale pilot extraction services to meet our clients' research needs at the initial stages. These pilot services help clients evaluate our processes and product quality to ensure that the desired results are achieved before proceeding to large-scale production.
Q: Do you provide storage recommendations for extracellular vesicles?
A: Yes, we offer detailed storage recommendations for extracellular vesicles to our clients. We can also provide lyophilization (freeze-drying) services to maintain the product's activity and stability.
References
-
Almeria, C.; et al. Heterogeneity of mesenchymal stem cell-derived extracellular vesicles is highly impacted by the tissue/cell source and culture conditions. Cell and Bioscience. 2022, 12(1):51.
-
Bahr, MM.; et al. Preservation techniques of stem cells extracellular vesicles: a gate for manufacturing of clinical grade therapeutic extracellular vesicles and long-term clinical trials. International Journal of Veterinary Science and Medicine. 2020, 8(1):1-8.
-
Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only. Cannot be used by patients.
Related Services:

 Fig.1 Key considerations for MSC-derived EV production.1,3
Fig.1 Key considerations for MSC-derived EV production.1,3
 Fig.2 Schematic diagram of freeze drying.2.3
Fig.2 Schematic diagram of freeze drying.2.3









