Lung-Targeted Exosome Modification Service
Overview Services Features FAQs
Creative Biolabs has long accumulated the most specialized project experience and technology to enhance the combination of universal delivery ability and specific targeting of exosomes. In particular, we offer research services to facilitate exosome-targeted lung modification.
Exosome Lung-targeting Specificity
It is indispensable that the organ-specific distribution of exosomes has an impact on the effective action of exosome-delivered cargoes. With the presence of specific proteins such as CD47 on the surface, exosomes can avoid phagocytosis and receptor-mediated endocytosis by mononuclear macrophages and exhibit significantly better circulating stability than other carriers. However, the organ-specific distribution of exosomes is accompanied by different characteristics of surface integrin expression, which raises the challenge of enrichment of exosomes toward target regions.
Exosomes produced using sophisticated biomolecular engineering methods have become highly promising drug delivery vehicles. In particular, it is worth noting that their organ-targeting specificity plays a key role in the distribution and release of cargo after systemic administration.
Proteomics-based studies of exosomal integrin expression patterns have identified a convergence of integrin α6β4 and integrin α6β1-targeted exosomes in the lung. This directed migration involves the recruitment and binding of integrins to raft-localized C4.4 A, MT1-MMP, allowing for a shift in adhesion and motility. Moreover, knockdown of integrin α6β4 showed reduced access to exosomes by lung cells. Similarly, through the investigation of the exosome's mediating role in the metastasis of multiple tumors and their settlement in distant organs, marker molecules targeting the lung are continuing to be discovered. These not only provide clues to the role of exosomal integrins in the propensity of tumor metastasis, but also provide new ideas for the modification and production of exosome delivery vectors for lung targeting.
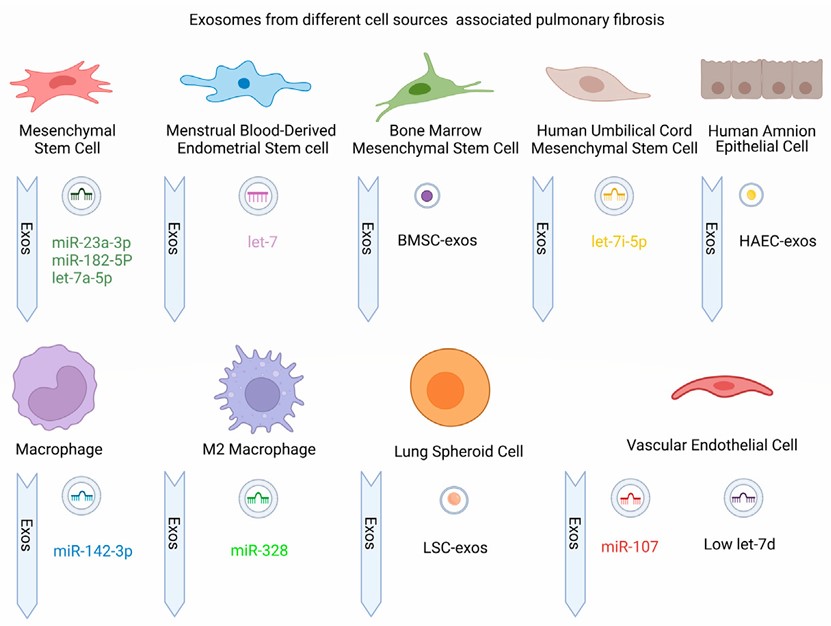 Fig. 1 Regulatory exosomes from different sources in pulmonary fibrosis.1
Fig. 1 Regulatory exosomes from different sources in pulmonary fibrosis.1
Exosome Modifications Targeting the Lung at Creative Biolabs
The deeply modifiable nature of exosomes confers the possibility to manipulate their molecular composition and to confer new desired targeting properties. In the development and application of exosome delivery vectors, Creative Biolabs can not only genetically engineer the use of donor cells to obtain exosomes displaying lung-targeting peptides on their surface, but also exogenously enhance the lung-targeting ability of engineered exosomes through ligand modifications. The ligand modification is performed with the help of multiple membrane proteins as anchoring scaffolds and intermolecular linkage with the help of click chemistry. Based on this lung-targeting engineering modification strategy, it becomes possible to modify, purify and isolate exosomes for specific lung tissue/cellular targeting using molecular biotechnology and exosome isolation platforms.
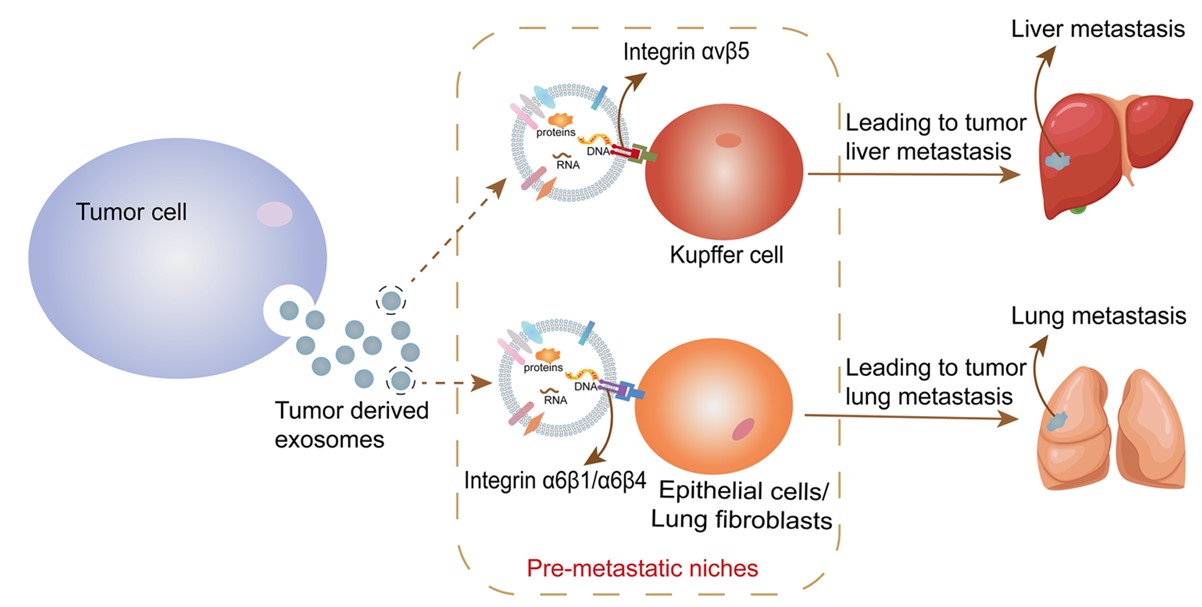 Fig. 2 Exosomal membrane protein integrin induces its targeting metastasis.2
Fig. 2 Exosomal membrane protein integrin induces its targeting metastasis.2
Features
-
Precision Targeting: Our Lung-Targeted Exosome Modification service utilizes state-of-the-art technology to precisely target exosomes to the lungs, ensuring efficient delivery of therapeutic cargo to the desired site.
-
Customized Modification: We offer tailored modifications to exosomes based on your specific research needs, including surface engineering for enhanced targeting and payload loading for optimized therapeutic efficacy.
-
Quality Control: We adhere to rigorous quality control standards throughout the modification process to ensure the integrity and functionality of the lung-targeted exosomes.
FAQs
Q: How can Lung-Targeted Exosome Modification benefit my research?
A: Lung-Targeted Exosome Modification can enhance the targeted delivery of therapeutic cargo to the lungs, making it an ideal tool for studying lung-related diseases or developing novel therapeutic strategies for lung disorders.
Q: Can you customize the modification of exosomes for specific cargo delivery to the lungs?
A: Yes, our service offers customized modification of exosomes to optimize cargo delivery to the lungs. We can tailor the surface properties and loading capacity of exosomes based on your specific research requirements.
Q: How do you ensure the efficiency and specificity of lung targeting with modified exosomes?
A: We employ advanced techniques and technologies to ensure the precision targeting of exosomes to the lungs. Our team of experts carefully designs and validates the modification strategy to achieve efficient and specific delivery of exosomes to the lung tissue.
Creative Biolabs has built a proven system for lung-targeted exosome customization with the support of a top-notch team of expert scientists at every step of exosome modification and analysis. We can flexibly customize and produce service solutions containing specific cargoes of engineered modified lung-targeted exosomes as clients require. Please feel free to contact us to advance your research.
References
-
Yang, Yang, et al. "Exosomes in pathogenesis, diagnosis, and treatment of pulmonary fibrosis." Frontiers in Pharmacology 13 (2022): 927653. Under Open Access license CC BY 4.0. The image was modified by extracting and using only the part related to exosomes from different cell sources in PF from the original image, and the title was revised accordingly.
-
He, Jiao, et al. "Exosomal targeting and its potential clinical application." Drug Delivery and Translational Research 12.10 (2022): 2385-2402. Under Open Access license CC BY 4.0, without modification.
For Research Use Only. Cannot be used by patients.
Related Services:

 Fig. 1 Regulatory exosomes from different sources in pulmonary fibrosis.1
Fig. 1 Regulatory exosomes from different sources in pulmonary fibrosis.1
 Fig. 2 Exosomal membrane protein integrin induces its targeting metastasis.2
Fig. 2 Exosomal membrane protein integrin induces its targeting metastasis.2









