Circulating Endothelial Progenitor Cells (CEPCs)-Targeted Exosome Modification Service
Overview Services Features FAQs
Creative Biolabs has introduced an innovative engineered exosome modification platform for delivery to circulating endothelial progenitor cells (CEPCs), allowing improved targeting to CEPCs by investigating donor cells and modifying exosome surface molecules.
Overview of CEPCs and Their Role in Vascular Endothelial Repair
CEPCs are found in the peripheral blood circulation and are strongly capable of proliferating, migrating and differentiating into mature vascular endothelial cells. Since being involved in the process of vascular neogenesis and repair after endothelial injury, CEPCs have become a research focal area in vascular tissue engineering, in vivo ischemic tissue, and vascular neogenesis of tumor tissue. As precursor cells of mature endothelial cells, CEPCs constitute an essential resource for damaged vascular endothelial cell repair in various cardiovascular diseases. Specifically, local vascular injury stimulates CEPCs to migrate home and accelerate vascular re-endothelialization, while pathological neointima formation is reduced. The key biological basis for the repair of damaged vascular endothelium and treatment of related diseases is the drug delivery system to mobilize effective CEPCs.
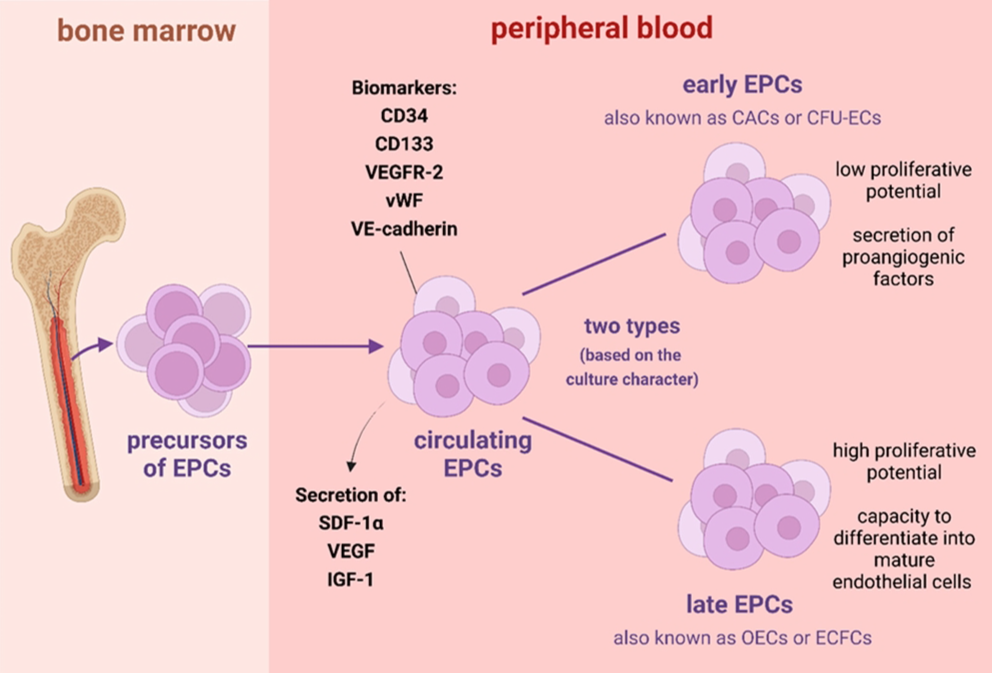 Fig. 1 Characteristics of endothelial progenitor cells.1
Fig. 1 Characteristics of endothelial progenitor cells.1
Exosome Modifications Targeting the CEPCs at Creative Biolabs
-
Exosome modification strategies
The most common technique for exosome modification is the construction of exosomal surface target molecules that recognize specific target receptors. Insertion of target proteins or their associated plasmids fused to exosomal membrane proteins in donor cells and then exosomes containing targeting peptides can be isolated from donor cells in post-secreted cultures. For enhancing the exosome-displayed peptide stability, the addition of a glycosylation motif at the N terminus is under consideration. Common strategies for exosome display include resorting to devices such as membrane protein LAMP-2B, CD63, and click chemical conjugation. In addition, exosomes have a natural preference for specific targeting influenced by the donor to express specific peptides and cell adhesion molecules. The capture of exosomes by specific types of recipient cells is also subject to the appropriate choice of donor source. Therefore, the engineering modification of exosomes with klotho proteins is promising for targeting CEPCs.
-
Engineered exosome services for targeting CEPCs
Exosome-mediated drug delivery with low toxicity, low immunogenicity, and high engineerability is used as a vehicle for the delivery of cell-free therapies. In addition, the finding that klotho gene variants are accompanied by reduced peripheral blood CEPCs indicates that the close relationship between exploring klotho proteins and CEPCs contributes to a greater understanding and treatment of diseases related to vascular endothelial injury.
Klotho proteins are designed to target CEPCs with engineering exosome modifications involving the co-receptor formation process of endocrine fibroblast growth factor (FGF). Overexpression of klotho plasmids in exosome donor cells followed by encapsulation of other drugs and/or molecules such as siRNA in the secreted exosomes has been shown to capture and endocytose klotho-modified exosomes in CEPCs. Those exosomes eventually disassemble free klotho proteins and drugs, mobilizing CEPCs to maintain patency and exert good therapeutic properties of endothelialization in chemotaxis to the site of injury.
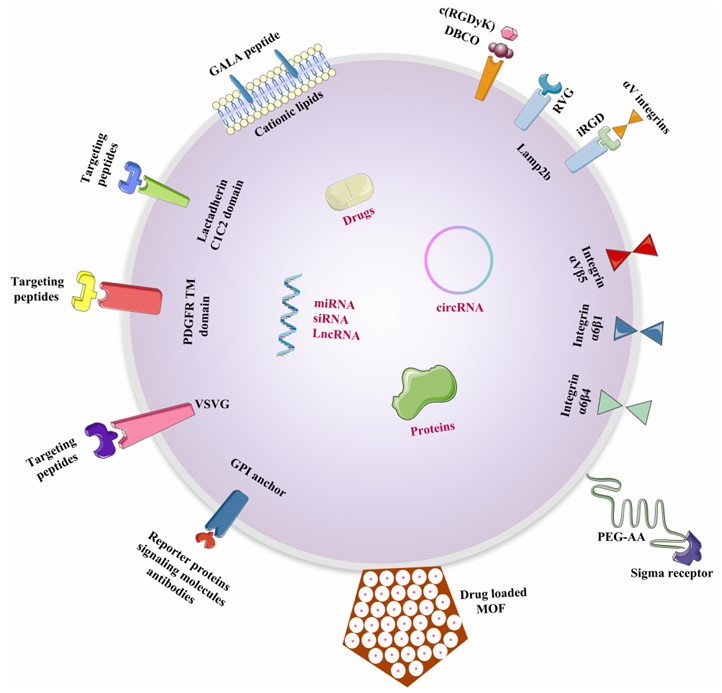 Fig. 2 Methods of loading different cargos to specific tissues through engineered exosomes.2
Fig. 2 Methods of loading different cargos to specific tissues through engineered exosomes.2
Features
-
Precision Targeting: Our service offers precise modification of exosomes to specifically target CEPCs, ensuring maximum efficacy and minimal off-target effects.
-
Enhanced Therapeutic Potential: Modified exosomes exhibit enhanced therapeutic potential by delivering cargo directly to CEPCs, promoting regenerative processes, angiogenesis, and tissue repair.
-
Tailored Cargo Delivery: We customize exosome cargo to carry therapeutic molecules, including nucleic acids, proteins, or small molecules, tailored to the unique requirements of CEPCs-based therapies.
-
Advanced Bioengineering Techniques: Leveraging cutting-edge bioengineering techniques, we engineer exosomes with high precision, optimizing their stability, targeting efficiency, and therapeutic payload capacity.
FAQs
Q: What are CEPCs, and why are they targeted for modification?
A: CEPCs are a subset of progenitor cells with the capacity to promote vascular repair and angiogenesis. Targeting CEPCs for exosome modification offers a promising strategy to enhance therapeutic outcomes in cardiovascular diseases, ischemic conditions, and tissue regeneration.
Q: How are exosomes modified to target CEPCs specifically?
A: Exosomes are modified through various techniques such as surface functionalization or genetic engineering to display targeting ligands or peptides that recognize receptors specifically expressed on CEPCs. This ensures the selective uptake of modified exosomes by CEPCs, maximizing therapeutic efficacy.
Q: How do I know if this service is suitable for my research needs?
A: Our team of experts is available to discuss your research goals and provide tailored recommendations. Whether you are studying cardiovascular diseases, tissue regeneration, or angiogenesis, we can assist you in designing a customized exosome modification strategy to meet your specific requirements.
Since the precise delivery patency of natural exosomes remains unsatisfactory due to their poor targeting, Creative Biolabs has established a platform for the modification and production of exosomes modified with target proteins or target antigens. The capture of specific receptor cells with exosomes is conferred with modified surface capture molecules. It enables the precise targeting of exosomal carriers with loaded cargo to realize the requirements. Please contact us with your interest.
References
-
Rudnicka-Drożak, Ewa, et al. "Endothelial Progenitor Cells in Neurovascular Disorders—A Comprehensive Overview of the Current State of Knowledge." Biomedicines 10.10 (2022): 2616. Under Open Access license CC BY 4.0. The image was modified by revising the title.
-
Xu, Mengqiao, et al. "Recent advancements in the loading and modification of therapeutic exosomes." Frontiers in Bioengineering and Biotechnology 8 (2020): 586130. Under Open Access license CC BY 4.0. The image was modified by revising the title.
For Research Use Only. Cannot be used by patients.
Related Services:

 Fig. 1 Characteristics of endothelial progenitor cells.1
Fig. 1 Characteristics of endothelial progenitor cells.1
 Fig. 2 Methods of loading different cargos to specific tissues through engineered exosomes.2
Fig. 2 Methods of loading different cargos to specific tissues through engineered exosomes.2









