Targeting Tumors and Microenvironment of Multi-antibody-modified Exosome Service
Exosomes are vesicle-shaped natural biological particles produced by cells with unique transport pathways and biological functions, which can be used to create new dosage forms for precise delivery to tumors and their TME (tumor microenvironment) in the complex in vivo environments and enhance the expected efficacy of the loaded drugs, with promising applications in the field of individualized tumor intervention. Creative Biolabs has established the exosome production modified with multi-specific antibodies and research service of targeting tumors and their TME to achieve dual targeting, thereby enhancing the intratumoral retention of the loaded drugs and improving the immune TME, which brings new ideas for engineering exosome-based modifications and tumor interventions through the above-mentioned synergistic mechanisms.
Introduction
The surface of exosome membranes has been modified by multi-specific antibodies that combine antibodies targeting several tumor-positive antigens and antibodies targeting TMEs, allowing them to display unique tissue-active selectivity and largely influence their biodistribution through ligand-receptor mediation. Previous studies have often focused on the high expression of epidermal growth factor receptor EGFR and Her2 in a variety of tumor tissues. In addition, the tumor stroma has been increasingly recognized as an important player in tumorigenesis, drug resistance, angiogenesis, and metastasis, with CAF (cancer-associated fibroblasts) being one of the stromal components. FAP (fibroblast activation protein) is involved in the regulation of the extracellular matrix as a marker of CAF. Thus, several of the constituent multi-specific antibodies of anti-EGFR, anti-Her2-II, anti-Her2-IV, and anti-FAP have been selected for attachment to the surface of exosomes, leading to sequential uptake of the drug by the tumor and its TME and improving its chemotherapeutic effect on antigen-positive cancer cells.
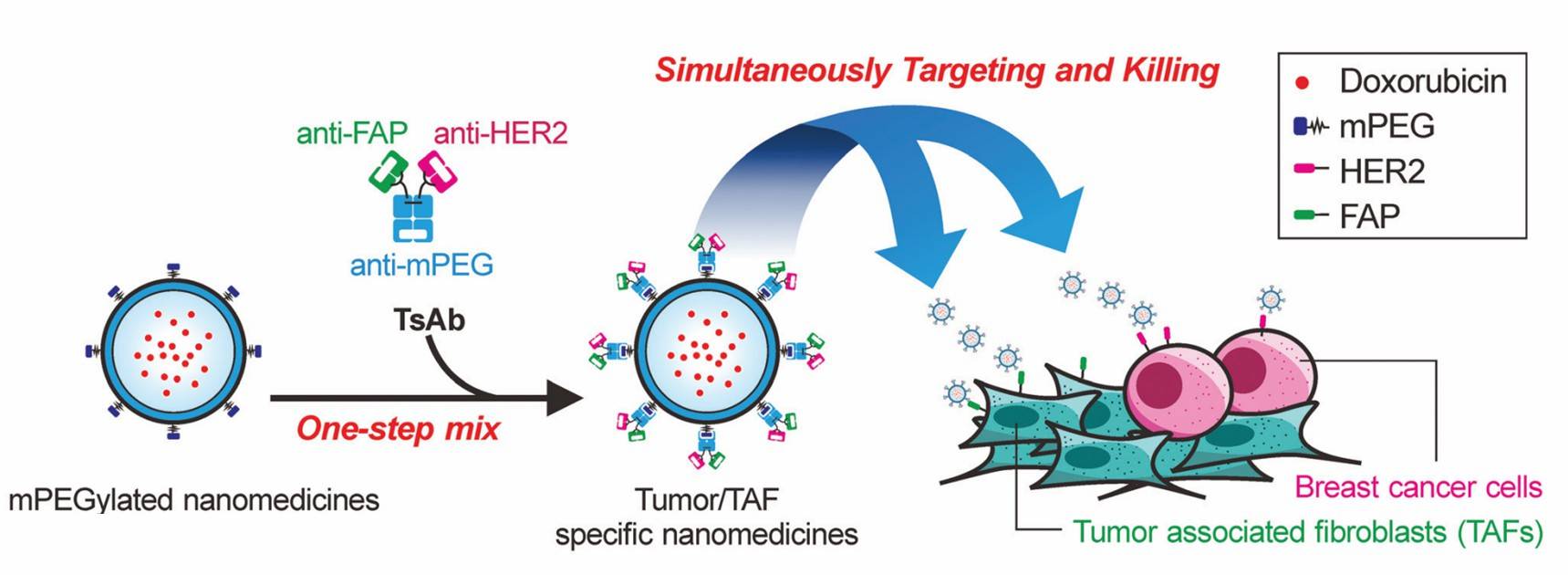 Fig.1 Multi-specific antibody-modified exosomes for simultaneous elimination of tumors containing TAFs. (Chen, 2021)
Fig.1 Multi-specific antibody-modified exosomes for simultaneous elimination of tumors containing TAFs. (Chen, 2021)
Exosome-Engineered Modification Strategies and Features
It has been discovered that fusing anti-PEG scFv with the C-terminus of antibodies that target tumor antigens results in multi-specific antibodies that maintain the initial efficacy of the targeting antibodies while actively luring drug-loaded exosomes to congregate at the tumor location. Further, a one-step pre-constructed multi-specific antibody containing anti-PEG, anti-FAP, and targeted tumor antigen was able to self-assemble on the exosome surface, effectively triggering endocytosis, overcoming the delivery barrier of antitumor drugs and significantly enhancing the cytotoxicity of exosome-loaded drugs. The modification conditions of this PEG-based non-covalent coupling strategy are relatively milder and universal compared to covalent coupling approaches such as click chemistry. Not limited to the above means of production, Creative Biolabs also offers services for the exogenous loading of cargo into exosomal delivery vehicles, enabling high drug delivery efficiency while also preserving the function and integrity of exosomes and drugs. Our advanced engineering technologies are divided into pre-secretory and post-secretory loading methods to efficiently load drugs into exosomes without disrupting the integrity of the exosome membrane.
Tumor immunotherapy, which mobilizes the organism's immune cells to recognize and kill tumors, is a highly promising new strategy for tumor treatment. Building on its current focus on enhancing the function and number of immune cells in the body, Creative Biolabs offers exosome production, cargo loading, and functional investigation services for multi-specific antibody modifications targeting tumors and TME. Such exosomes actively overcome the problem of low drug penetration in solid tumor sites and their immunosuppressive microenvironment, allowing their loading of drugs to effectively perform anti-tumor functions. Please contact us to discuss your project.
Reference
-
Chen, M.; et al. A novel anti-tumor/anti-tumor-associated fibroblast/anti-mPEG tri-specific antibody to maximize the efficacy of mPEGylated nanomedicines against fibroblast-rich solid tumor. Biomater Sci. 2021, 10(1): 202-215.
For Research Use Only. Cannot be used by patients.
Related Services:

 Fig.1 Multi-specific antibody-modified exosomes for simultaneous elimination of tumors containing TAFs. (Chen, 2021)
Fig.1 Multi-specific antibody-modified exosomes for simultaneous elimination of tumors containing TAFs. (Chen, 2021)









