Enhancing Tumor Phagocytosis of Multi-antibody-modified Exosome Service
Macrophage-based tumor immunotherapy achieves effective interference with tumor progression. The application of multi-specific antibody-modified exosomes that promote phagocyte contact, encapsulation, and phagocytosis of tumor cells further complements exosome engineering strategies. Creative Biolabs offers manufacturing and validation services for multi-specific antibody-modified exosomes that enhance tumor phagocytosis, helping clients for producing high-quality desired exosomes.
Introduction of Multi-Specific Antibody-Modified Exosomes for Enhanced Tumor Phagocytosis
Enhanced macrophage digestion of cancer cells by targeting phagocytosis-related regulatory molecules has been confirmed by various evidence, such as the inhibitory immune checkpoint CD47/SIRPα (signaling regulatory protein α) and the pro-phagocytic uptake molecule calreticulin, exerting effective anti-tumor effects. CD47, as a transmembrane glycoprotein widely highly expressed in tumor cells, also known as integral protein-associated protein, inhibits phagocytosis of macrophages by binding to the N-terminal of inhibitory immune receptor SIRPα on immune cells, enabling the escape of tumor cells to be destroyed by the immune system. As one of the most important inhibitory immune checkpoints, blockade of the CD47-SIRPα signaling pathway effectively activates phagocytosis of tumor cells by macrophages and downstream antitumor immune responses, which can be achieved with exosomes co-expressing anti-CD47 and anti-SIRPα. Calreticulin is an endoplasmic reticulum-resident protein that supports normal antigen presentation on MHC class I molecules to ensure dedicated phagocytic uptake of pathogens. Modification of calreticulin on exosomes activates aggregation of phagocytic signals near tumor cells and enhanced phagocytosis by binding to LRP1 on the surface of macrophages. This strategy to stimulate innate immune effects can also be combined with strategies to enhance the adaptive immune system, such as the construction of bispecific antibody-modified exosomes co-expressing anti-PD1 and anti-CD47 exhibiting the ability to first induce phagocytosis to eat cancer cells and then use these oncogenic components to stimulate adaptive immunity of T cells to exert continuous anticancer properties.
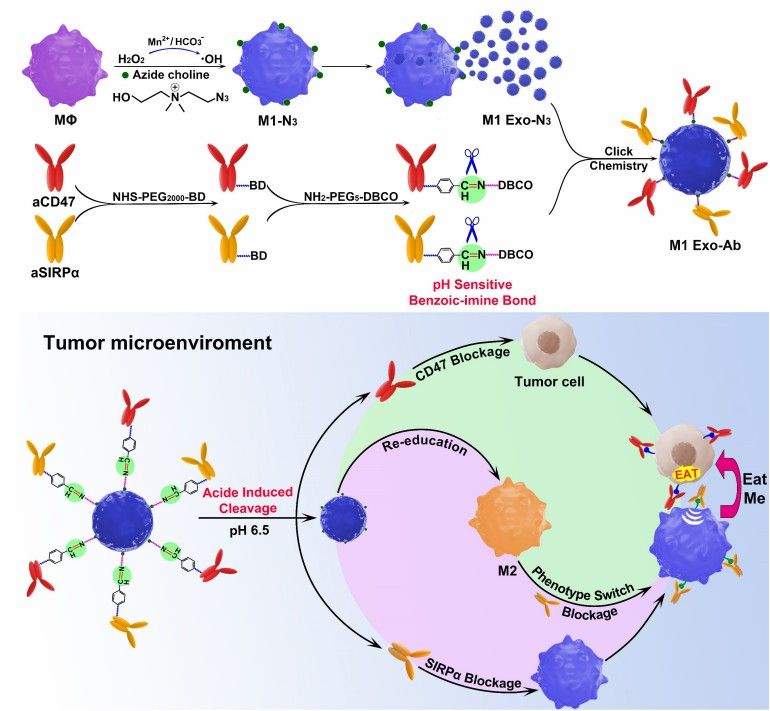 Fig.1 The synergistic anticancer effect of M1 Exo engineered with aCD47 and aSIRPα. (Nie, 2020)
Fig.1 The synergistic anticancer effect of M1 Exo engineered with aCD47 and aSIRPα. (Nie, 2020)
Strategies to Enhance Tumor Phagocytosis with Multi-specific Antibody-modified Exosomes
Bridging these phagocytosis-enhancing multi-specific antibodies to the exosome surface by click chemistry or PEG is a well-established strategy. Specifically, a combination of the above-mentioned pro-phagocytosis antibodies and antibodies targeting the tumor are selected and pegged with dibenzocyclooctyne moieties to the multi-specific antibodies. Simultaneous modification of the exosomes with azide enables the coupling of the multi-specific antibodies to the exosomes based on click chemistry. This form of antibody has a higher potency compared to its free form due to the protection of the exosomal lipid membrane. In addition, the selection of exosome-producing cells with natural immune-activating effects, such as M1-type macrophages or dendritic cells, can further enhance the reversal of the immunosuppressive microenvironment and the stimulation of T cells by exosomes. This simultaneously achieves a combined antitumor effect of enhanced phagocytosis, enhanced T-cell toxicity, and deregulation from the immunosuppressive tumor microenvironment.
Research on the application of multi-specific antibody-modified exosomes that enhance systemic phagocytosis of cancer cells has inspired new ideas for exosome engineering. Such multi-specific antibody-modified exosomes can target both tumor antigens and macrophage surface antigens to enhance the possibility of tumor phagocytosis. Creative Biolabs offers a well-established service for the production and functional exploration of pro-phagocytosis multi-specific antibody-modified exosomes. Please contact us with your interest.
Reference
-
Nie, W.; et al. Responsive exosome nano-bioconjugates for synergistic cancer therapy. Angew Chem Int Ed Engl. 2020, 59(5): 2018-2022.
For Research Use Only. Cannot be used by patients.
Related Services:

 Fig.1 The synergistic anticancer effect of M1 Exo engineered with aCD47 and aSIRPα. (Nie, 2020)
Fig.1 The synergistic anticancer effect of M1 Exo engineered with aCD47 and aSIRPα. (Nie, 2020)









