Tumor Cells-Targeted Exosome Modification Service
Overview Services Features FAQs
Designing exosomes to carry specific ligands and specific proteins allows the investigation of exosome-mediated drug transport and its effects on tumor lesions. With a deep comprehension of exosome modification and production, Creative Biolabs offers clients exosome modification services for targeting tumors.
Exosome Distribution in Tumors
-
It has been demonstrated that exosomes with natural lipid membrane structure and 100 nm size exhibit enhanced EPR effects in tumors.
-
Not only are exosomes exposed to organ-selective ligands capable of recognizing tumor integrins, but are also covered with glycans that affect tissue distribution. For example, glioblastoma-derived exosomes carrying glycosaminoglycans are directed to CCR8-positive glioblasts by bridging CCL8.
-
High levels of sphingomyelin and gangliosides attributed to the membrane turned positively charged in the acidic tumor microenvironment (TME), enhancing exosome uptake by tumors compared to physiological conditions.
However, the higher inter-tissue osmolarity, inadequate blood perfusion, and the risk of metastasis in solid tumors still pose challenges to the exosome delivery of therapeutic agents. Benefiting from exosomes' high modifiability for targeted delivery is a blessing for the future of medical oncology, especially for tumors that are only diagnosed at advanced stages and have a poor prognosis.
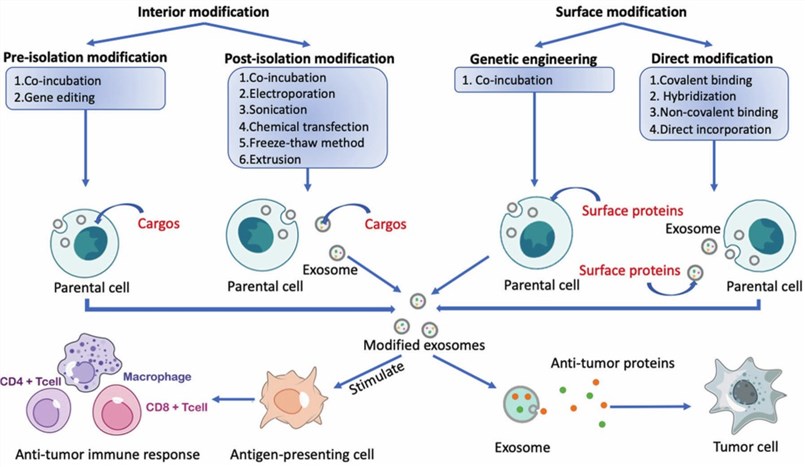 Fig.1 The internal and surface protein modification of exosomes.1, 3
Fig.1 The internal and surface protein modification of exosomes.1, 3
Exosome Modification Services for Tumor Cells at Creative Biolabs
-
Passive-Targeted Exosome Modification
Effective passive targeting facilitates the enrichment of systemically administered exosomes in tumor tissue, a prerequisite for subsequent exosome targeting to tumor-specific cells.
|
Rational
|
Strategies
|
|
Prolonging circulation time
|
-
Overexpression of the surface anti-phagocytic cytokine CD47 protects exosomes from phagocytosis and clearance, thus significantly increasing the circulation time.
-
Surface PEG conjugation results in long-circulating exosomes and improves their stability.
|
|
Increasing tumor infiltration
|
-
Membrane-modified RGD interacts with the vascular target integrin αvβ3, a cell-penetrating peptide applied to tumor targeting in a cytosolic-independent penetration manner.
-
The dual function of exosomes for tumor homing and membrane penetration is achieved with surface modification of tLyp-1-mediated interaction with NRP-1, which is widely present in endothelial cells and TME.
|
Creative Biolabs offers two types of exosome modification services, pre-isolation, and post-isolation, based on the modification time. Donor cells are transfected with plasmids containing ligand sequences before exosome isolation from the cells resulting in the exosome being produced with the corresponding ligands displayed on its surface. In post-isolation, the ligands are allowed to be directly modified on the exosome surface by conjugation reactions or click chemistry.
-
Active-Targeted Exosome Modification
Several modifiable proteins are available for exosome active targeting applications, including antibodies, peptides, protein-based ligands, nucleic acid-based ligands, and small molecules.
|
Rational
|
Strategies
|
|
Restore TME normalization
|
-
Immune responses disrupted in TME by T-cell depletion can be relieved by constructing dual-targeted exosomes with anti-EGFR and anti-CD3.
-
Exosomes with Lewis antigen modified by rocket algae glycosylated glycans show enhanced dendritic cell uptake and mediated immune activation.
-
Methotrexate was demonstrated on the exosome surface to act as a ligand binding to tumor-associated macrophages with high folic acid receptor-2 expression.
|
|
Selectively taken up by cancer cells
|
-
GE11 peptide targets EGFR overexpressing tumors, while the R8 peptide stimulates the exosome uptake by tumor cells.
-
Non-peptide ligands AS1411 targeting tumor cells overexpressing nucleophosmin and Sgc8 targeting protein tyrosine kinase 7 positive tumors.
|
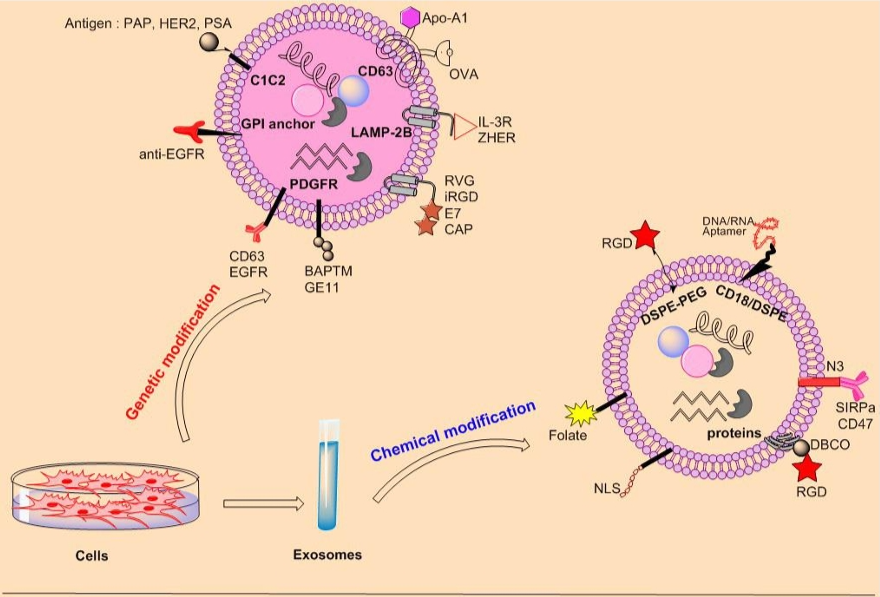 Fig. 2 The functionalization of exosome surface.2, 3
Fig. 2 The functionalization of exosome surface.2, 3
Features
-
Highly Specific Targeting: This service utilizes cutting-edge bioengineering techniques to enable exosomes to identify and target specific tumor cells accurately. Through surface modification or carrier design, we enhance the exosome's affinity and invasion capability towards tumor tissue, minimizing the impact on normal cells.
-
Customized Carrier Design: We provide customized exosome carrier designs to fulfill our client's unique requirements. This includes regulating cargo load, designing release dynamics, and implementing synergistic loading strategies to improve targeting efficacy.
-
Optimized Biodistribution and Pharmacokinetics: By modifying the physical and chemical properties of exosomes, we optimize their distribution within the body, extend their circulation time in the bloodstream, and enhance their ability to penetrate the tumor microenvironment.
-
Safety and Biocompatibility: Exosomes are modified under stringent biosafety conditions to ensure they meet quality and safety standards.
-
Multi-Level Functional Verification: Our service includes comprehensive functional verification from the molecular level to preclinical models, ensuring products meet the expected targeting effectiveness and biological activity.
As an advanced professional organization in the exosome research and application field, Creative Biolabs has accumulated extensive insight into various types of tumors and research projects applying exosome-targeted delivery. The exosome modification strategy for tumor targeting comprehensively takes into account factors including passive enrichment in tumor foci and selective internalization in specific pathological cells to optimize exosome targeting. Our experts specialize in providing customized exosome modification services for targeting tumors to meet the client's particular needs. Please contact us to discuss your project.
FAQ
Q: Which tumor kinds are eligible for this service's targeting?
A: Tumor cells-targeted exosome modification can be applied to various types of tumors, including solid tumors (e.g., breast cancer, lung cancer, colorectal cancer) and hematological malignancies (e.g., leukemia, lymphoma). By tailoring exosomes to tumor cell-specific markers or microenvironments, targeted therapies can be developed for different cancer types.
Q: How does exosome modification target tumor cells?
A: Exosome modification targets tumor cells by incorporating specific ligands or antibodies on the exosome surface that recognize and bind to receptors unique to tumor cells. This targeted approach ensures that the exosomes preferentially home in on tumor cells, minimizing off-target effects and improving the delivery of agents.
Q: Are there any safety concerns with using modified exosomes?
A: While exosome-based therapies hold great promise, safety is a critical consideration. Potential concerns include immunogenicity, off-target effects, and the long-term impact of exosome-based delivery. Rigorous preclinical and clinical testing is conducted to ensure the safety and efficacy of modified exosomes.
References
-
Wang, Xinyi, et al. "The updated role of exosomal proteins in the diagnosis, prognosis, and treatment of cancer." Experimental & Molecular Medicine 54.9 (2022): 1390-1400.
-
Akbari, Ali, et al. "Engineered Exosomes for Tumor-Targeted Drug Delivery: A Focus on Genetic and Chemical Functionalization." Pharmaceutics 15.1 (2022): 66.
-
Under Open Access license CC BY 4.0. The image was modified by revising the title.
For Research Use Only. Cannot be used by patients.
Related Services:

 Fig.1 The internal and surface protein modification of exosomes.1, 3
Fig.1 The internal and surface protein modification of exosomes.1, 3
 Fig. 2 The functionalization of exosome surface.2, 3
Fig. 2 The functionalization of exosome surface.2, 3









