Immune cell function is regulated by co-suppression and costimulatory molecules. It is well known that two promising cancer immunotherapeutic agents, programmed cell death protein 1 (PD1) and cytotoxic T lymphocyte protein 4 (CTLA4), are mainly composed of antagonist antibodies that block immunological checkpoints. Looking forward, there are great promises with agonist antibodies that target costimulatory receptors, and more and more of these drugs are undergoing developmental stages.
Creative Biolabs‘ scientists are constantly on the lookout for advances in the development of antagonist antibodies. We are committed to studying key considerations and potential defects in the design and development of antagonist antibodies, which enable us to develop antagonist antibodies for the clinical development of cancer therapy for our clients.
Despite years of research, melanoma remains a major challenge for modern medicine. Here, we quote the latest report on melanoma therapy in 2019. These data are derived from clinical trials registered in the International Clinical Trial Registration Platform (ICTRP), one of the largest databases of clinical trials. The diagram below shows the most common interventions in the ICTRP database from 1971 to 2018:
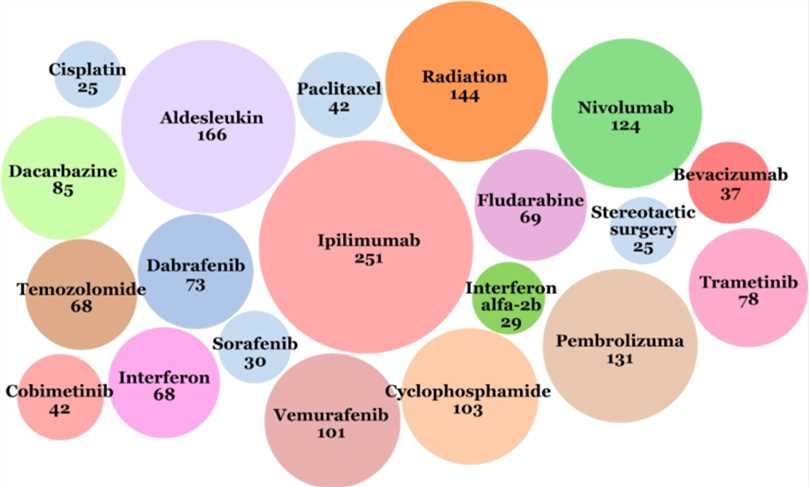 Fig.1 Interventions that most often appeared in the ICTRP database from 1971 to 2018. (Sonia, 2019)
Fig.1 Interventions that most often appeared in the ICTRP database from 1971 to 2018. (Sonia, 2019)
Eleven interventions were presented in the database, including antibodies, vaccines, interleukins, and combination products. Among them, the most commonly used interventions in melanoma clinical trials are ipilimumab (immunotherapy checkpoint antagonist antibody, targeting CTLA-4), pembrolizumab (immunotherapy checkpoint antagonist antibody, targeting PD-1), nivolumab (immunotherapy, checkpoint antagonist antibody, targeting PD-1). These data suggest strong rationality regarding the trend of melanoma antagonist antibody therapy.
Antibody-based drugs have increasingly been used to treat melanoma. Specifically, several small-molecule signaling antagonists approved by the FDA can target key oncogenic kinases and inhibit the progression of melanoma. Creative Biolabs‘ team of dedicated scientists provides customized antibody design and development services for the most advanced antagonists targeting melanoma. Examples of highlight targets include, but are not limited to:
| BRAF | MEK | PD-1 | PD-L1 | CTLA4 |
| B7-H1 | CSPG4 | VEGF | CD25 | IFNα2b |
Creative Biolabs has been working in the field of antibody engineering for more than a decade, and our scientists are fully confident of completing the most challenging antagonistic antibody development projects. For additional details for antagonistic antibody services, please for free to contact us.
Reference
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.