Introduction of CD3
CD3, also known as cluster of differentiation 3 or T-cell receptor complex, is a protein complex comprising four subunits (γ, δ, ε, and ζ) that associates with the T-cell receptor (TCR) on the surface of T cells. Encoded by five genes (CD3G, CD3D, CD3E, CD247, and CD3Z) located on different chromosomes in humans, CD3's structure consists of an extracellular immunoglobulin-like domain, a transmembrane region, and an intracellular tail with immunoreceptor tyrosine-based activation motifs (ITAMs). Crucial for T-cell development, activation, and signaling, CD3 transduces signals from the TCR to intracellular pathways upon recognition of antigen-presenting cells (APCs). This process leads to T-cell proliferation, differentiation, cytokine production, and cytotoxicity. As CD3 is universally expressed on all T cells, irrespective of their antigen specificity, it holds promise as a target for cancer immunotherapy. This property allows for the redirection of T cells to tumor cells expressing specific antigens.
Introduction of P-cadherin
P-cadherin, also known as cadherin-3 or placental cadherin, is a classical cell-to-cell adhesion molecule within the cadherin superfamily. Comprising five extracellular cadherin repeats, a single transmembrane domain, and a cytoplasmic tail interacting with catenins and other proteins, P-cadherin is encoded by the CDH3 gene on chromosome 16 in humans. Its structure is akin to other classical cadherins like E-cadherin and N-cadherin, albeit differing in amino acid sequence and glycosylation pattern. P-cadherin functions homeostatically in various normal tissues such as the basal layer of the epidermis, breast, prostate, and placenta. Through calcium-dependent homophilic interactions and intracellular signaling pathways, P-cadherin regulates cell differentiation, polarity, migration, and survival. Notably, P-cadherin becomes a potential target for cancer immunotherapy due to its aberrant or overexpression in solid tumors like breast, ovarian, prostate, endometrial, skin, gastric, pancreatic, and colon cancers. Its association with tumor aggressiveness, invasion, metastasis, stemness, and resistance to therapy enhances its appeal as an immunotherapeutic target.
Signaling Pathways Involved in Bispecific Antibodies Targeting CD3 and P-cadherin
BsAbs designed to target CD3 and P-cadherin initiate T cell activation by facilitating the cross-linking of CD3 with P-cadherin on tumor cells. This interaction induces the phosphorylation of immunoreceptor tyrosine-based activation motifs (ITAMs) in the CD3ζ subunits through Src family kinases (SFKs), including Lck and Fyn. Subsequently, the phosphorylated ITAMs recruit ZAP70 kinase, initiating the phosphorylation of LAT and SLP76 adaptor proteins. These proteins collectively form a signalosome that activates downstream signaling pathways. These pathways encompass PLCγ1, IP3R/Ca2+, PKCθ/NF-κB/IL-2R/STAT5/ERK1/2/MAPK/AP1/NFAT axes, regulating T-cell activation markers (e.g., CD69 and CD25), cytokine production (e.g., IFN-γ and TNF-α), cytotoxic molecules (e.g., perforin and granzyme B), and cell survival (e.g., Bcl-2 and Bcl-xL).
BsAbs targeting CD3 and P-cadherin additionally influence P-cadherin signaling in tumor cells by disrupting homophilic interactions, inducing internalization, or promoting degradation. These actions impact the stability of adherens junctions and activate intracellular signaling pathways, including β-catenin/Wnt/TCF/LEF/β-catenin/E-cadherin/Snail/EZH2 axes. These pathways govern crucial cellular processes such as cell adhesion, polarity, epithelial-mesenchymal transition (EMT), stemness, invasion, metastasis, proliferation, apoptosis, and drug resistance.
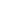
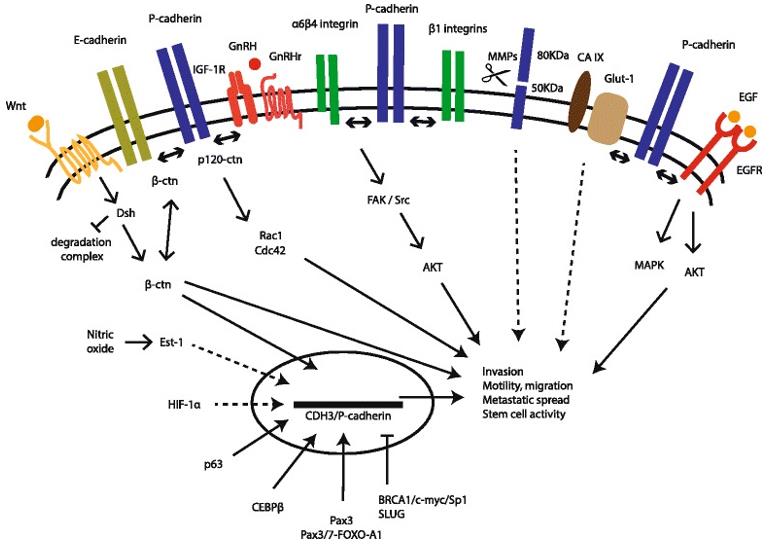 Fig.1 P-cadherin Signaling Pathways in the Malignant Setting (Vieira AF, 2015)
Fig.1 P-cadherin Signaling Pathways in the Malignant Setting (Vieira AF, 2015)
Clinic Status of Bispecific Antibodies Targeting CD3 and P-cadherin
As of now, no Bispecific antibodies (BsAbs) targeting CD3 and P-cadherin have received approval for clinical use. Nonetheless, several preclinical studies underscore the feasibility and efficacy of such BsAbs in diverse tumor models. For instance, PF-06671008, a dual-affinity re-targeting bispecific protein boasting an extended half-life, demonstrated robust T-cell-mediated cytotoxicity and tumor regression in models of P-cadherin-expressing breast, ovarian, prostate, endometrial, skin, gastric, pancreatic, and colon cancers. Table 1 provides a concise summary of relevant information about PF-06671008.
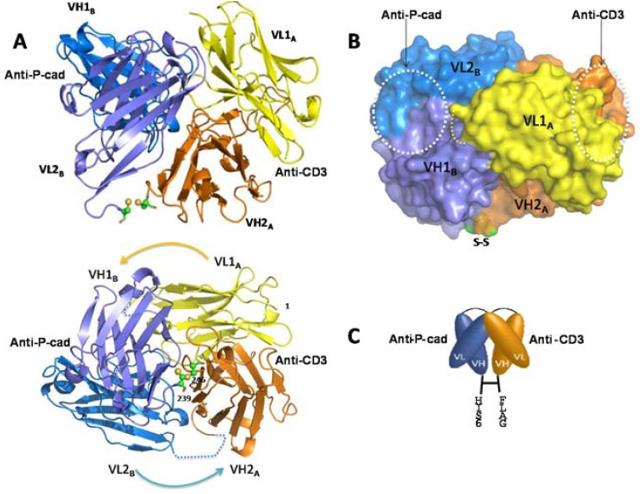 Fig.2 Crystallography of P-Cadherin x CD3 Bispecific Antibody (Root AR, 2016)
Fig.2 Crystallography of P-Cadherin x CD3 Bispecific Antibody (Root AR, 2016)
Table 1. Summary of PF-06671008
|
Property
|
Description
|
|
Name
|
PF-06671008
|
|
Type
|
Bispecific antibody
|
|
Format
|
Dual-affinity re-targeting bispecific protein
|
|
Targets
|
CD3 and P-cadherin
|
|
Mechanism of action
|
Redirects T cells to kill tumor cells expressing P-cadherin
|
|
Clinical status
|
Phase I clinical trial for advanced solid tumors
|
|
Preclinical efficacy
|
Potent T-cell-mediated cytotoxicity and tumor regression in various P-cadherin-expressing tumor models
|
|
Preclinical safety
|
Low immunogenicity and toxicity in animal studies
|
|
Pharmacokinetics
|
Extended half-life (~4.4 days in human FcRn knock-in mice)
|
|
Manufacturing
|
High expression (>1 g/L), high stability (Tm1 > 68 ℃), and robust purification properties (highly pure heterodimer)
|
References
-
Middelburg J, et al. Overcoming Challenges for CD3-Bispecific Antibody Therapy in Solid Tumors. Cancers (Basel). 2021 Jan 14;13(2):287.
-
Saber H, et al. An FDA oncology analysis of CD3 bispecific constructs and first-in-human dose selection. Regul Toxicol Pharmacol. 2017 Nov;90:144-152.
-
Crawford A, et al. Targeting Solid Tumors Using CD3 Bispecific Antibodies. Mol Cancer Ther. 2021 Aug;20(8):1350-1358.
-
van Roy F. Beyond E-cadherin: roles of other cadherin superfamily members in cancer. Nat Rev Cancer. 2014 Feb;14(2):121-34.
-
Vieira AF, et al. P-cadherin and the journey to cancer metastasis. Mol Cancer. 2015 Oct 6;14:178.
-
Paredes J, et al. P-cadherin expression in breast cancer: a review. Breast Cancer Res. 2007;9(5):214.
-
Harding JJ, et al. A Phase 1 Dose-Escalation Study of PF-06671008, a Bispecific T-Cell-Engaging Therapy Targeting P-Cadherin in Patients With Advanced Solid Tumors. Front Immunol. 2022 Apr 14;13:845417.
-
Fisher TS, et al. A CD3-bispecific molecule targeting P-cadherin demonstrates T cell-mediated regression of established solid tumors in mice. Cancer Immunol Immunother. 2018 Feb;67(2):247-259.
Our products and services are for research use only, and not for use in diagnostic or therapeutic procedures.
Welcome! For price inquiries, we will get back to you as soon as possible.
To order, please email
INQUIRY


 Fig.1 P-cadherin Signaling Pathways in the Malignant Setting (Vieira AF, 2015)
Fig.1 P-cadherin Signaling Pathways in the Malignant Setting (Vieira AF, 2015)
 Fig.2 Crystallography of P-Cadherin x CD3 Bispecific Antibody (Root AR, 2016)
Fig.2 Crystallography of P-Cadherin x CD3 Bispecific Antibody (Root AR, 2016)