Introduction of CD3
CD3, a protein complex and co-receptor of T cells, plays a pivotal role in activating both cytotoxic T cells (CD8+ naive T cells) and helper T cells (CD4+ naive T cells). Comprising four distinct chains—CD3γ, CD3δ, and two CD3ε chains—it forms associations with the T cell receptor (TCR) and transmits signals to activate T cells upon antigen recognition by the TCR. CD3 is universally expressed on all T cells, influencing T cell development, differentiation, and function. Its involvement in various immune-related diseases, including autoimmune conditions, transplant rejection, and cancer, establishes CD3 as a valuable target for immunotherapy.
Introduction of EGFRvIII
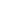
EGFRvIII represents a mutant variant of EGFR prevalent in various tumor types, including glioblastoma multiforme (GBM), breast cancer, head and neck cancer, and ovarian cancer. Originating from an in-frame deletion of 801 base pairs spanning exons 2–7 of the EGFR gene, this mutation results in a truncated extracellular domain devoid of the ligand-binding region. Characterized by constitutive tyrosine kinase activity, EGFRvIII activates multiple signaling pathways that bolster tumor cell survival, growth, and resistance. Notably, EGFRvIII is exclusively expressed in tumor cells and is correlated with heightened tumor aggressiveness, recurrence, and an unfavorable prognosis. Consequently, EGFRvIII emerges as a highly specific and crucial target for effective tumor therapy.
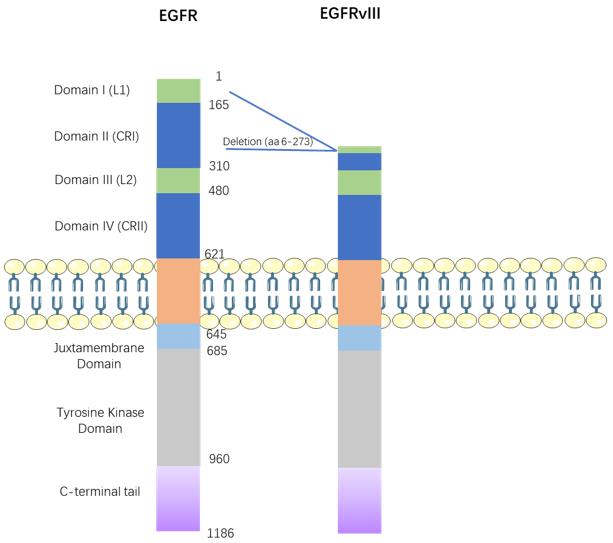 Fig.1 Functional Domains of EGFR and EGFRvIII
Fig.1 Functional Domains of EGFR and EGFRvIII
Signaling Pathways Involved in Bispecific Antibodies Targeting CD3 and EGFRvIII
The primary mechanism of action for bispecific antibodies targeting CD3 and EGFRvIII lies in bridging T cells with EGFRvIII-expressing tumor cells, inducing T cell activation, and promoting cytotoxicity against the tumor cells. Through binding to CD3, these antibodies mimic TCR signaling, activating T cell proliferation and effector functions. Simultaneously, by binding to EGFRvIII, bispecific antibodies hinder EGFRvIII signaling, inhibiting the survival and resistance mechanisms of tumor cells. This triggers multiple signaling pathways in both T cells and tumor cells, including PI3K/AKT, MAPK, NF-κB, and JAK/STAT. These pathways play diverse roles in regulating the cell cycle, differentiation, apoptosis, migration, and other processes in both T cells and tumor cells. For instance, the PI3K/AKT pathway, activated by both TCR and EGFRvIII signaling, promotes T cell survival and proliferation, along with tumor cell growth and invasion. The MAPK pathway, activated by both TCR and EGFRvIII signaling, modulates T cell differentiation and cytokine production, as well as tumor cell proliferation and angiogenesis. The NF-κB pathway, primarily activated by TCR signaling, enhances T cell activation and cytokine secretion, alongside promoting tumor cell survival and inflammation. The JAK/STAT pathway, mainly activated by EGFRvIII signaling, mediates tumor cell proliferation, migration, and resistance to apoptosis.
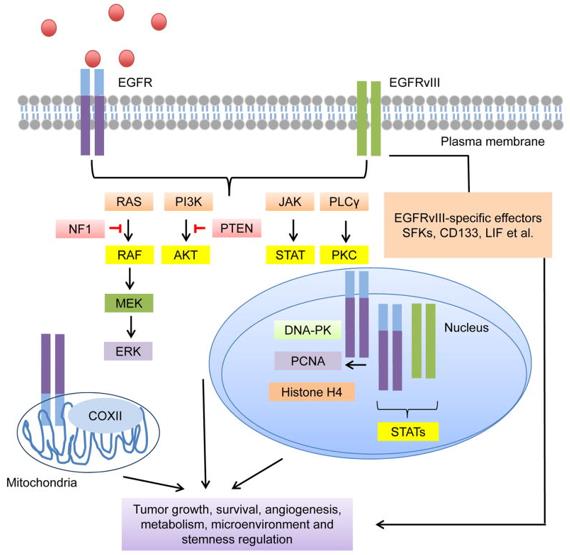 Fig.2 Signaling Pathways Mediated by EGFR/EGFRvIII (An Z, 2018)
Fig.2 Signaling Pathways Mediated by EGFR/EGFRvIII (An Z, 2018)
Clinic Status of Bispecific Antibodies Targeting CD3 and EGFRvIII
Currently, no bispecific antibodies specifically targeting CD3 and EGFRvIII have received marketing approval. However, multiple bispecific antibodies with this target are actively undergoing clinical trials, primarily focusing on treating glioblastoma multiforme (GBM) and other tumors expressing EGFRvIII.
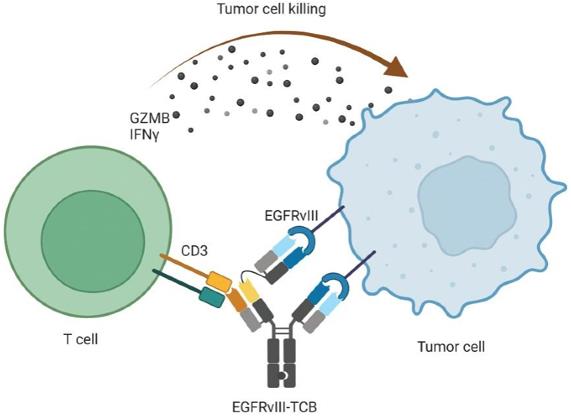 Fig.3 Schematic Diagram of the Mechanism of BsAbs Targeting CD3 and EGFRvIII (Iurlaro R, 2022)
Fig.3 Schematic Diagram of the Mechanism of BsAbs Targeting CD3 and EGFRvIII (Iurlaro R, 2022)
One promising candidate is AMG 596, a novel bispecific T-cell engager immuno-oncology therapy designed to target the tumor-specific antigen EGFRvIII and the T-cell co-receptor CD3. This innovative therapy aims to recruit and activate the patient's own T cells to eliminate tumor cells expressing EGFRvIII, a mutant form of EGFR prevalent in various tumors, including glioblastoma multiforme (GBM), the most common and aggressive primary brain tumor in adults. AMG 596 has demonstrated potent and specific anti-tumor activity in preclinical studies, leading to its advancement into phase I/II clinical trials for the treatment of EGFRvIII-expressing GBM. Distinguished as the first bispecific T-cell engager molecule specifically targeting EGFRvIII, AMG 596 holds promise as a groundbreaking immunotherapy for patients not only with GBM but also for those with other tumors expressing this antigen.
Table 1. Summary of AMG 596
|
Item
|
Information
|
|
Name
|
AMG 596
|
|
Type
|
Bispecific T-cell engager immuno-oncology therapy
|
|
Targets
|
CD3 and EGFRvIII
|
|
Mechanism of action
|
Bridges T cells with tumor cells expressing EGFRvIII, and induces T cell activation and cytotoxicity against the tumor cells
|
|
Clinical trial status
|
Phase I/II clinical trials for the treatment of EGFRvIII-expressing GBM
|
|
Preclinical data
|
Showed potent and specific anti-tumor activity in vitro and in vivo
|
|
Advantages
|
Highly specific and important target for tumor therapy; first bispecific T-cell engager molecule to target EGFRvIII; novel immunotherapy for GBM and other tumors that express EGFRvIII
|
|
NCT Number
|
NCT03296696
|
References
-
Middelburg J, et al. Overcoming Challenges for CD3-Bispecific Antibody Therapy in Solid Tumors. Cancers (Basel). 2021 Jan 14;13(2):287.
-
Singh A, et al. Overcoming the challenges associated with CD3+ T-cell redirection in cancer. Br J Cancer. 2021 Mar;124(6):1037-1048.
-
An Z, et al. Epidermal growth factor receptor and EGFRvIII in glioblastoma: signaling pathways and targeted therapies. Oncogene. 2018 Mar;37(12):1561-1575.
-
Gan HK, et al. The epidermal growth factor receptor variant III (EGFRvIII): where wild things are altered. FEBS J. 2013 Nov;280(21):5350-70.
-
Iurlaro R, et al. A Novel EGFRvIII T-Cell Bispecific Antibody for the Treatment of Glioblastoma. Mol Cancer Ther. 2022 Oct 7;21(10):1499-1509.
-
Gedeon PC, et al. A Rationally Designed Fully Human EGFRvIII:CD3-Targeted Bispecific Antibody Redirects Human T Cells to Treat Patient-derived Intracerebral Malignant Glioma. Clin Cancer Res. 2018 Aug 1;24(15):3611-3631.
-
Ellwanger K, et al. Highly Specific and Effective Targeting of EGFRvIII-Positive Tumors with TandAb Antibodies. Front Oncol. 2017 May 19;7:100.
-
Wang J, et al. A Novel EGFRvIII T-Cell Bispecific Antibody for the Treatment of Solid Tumors Expressing EGFR or EGFRvIII. Mol Cancer Ther. 2022 Oct;21(10):1499-1510.
-
Wang Z, et al. Bispecific Antibodies in Lung Cancer: A State-of-the-Art Review. Pharmaceuticals (Basel). 2021 Oct 7;16(10):1461.
-
Li D, et al. Development of a Novel Bispecific Antibody Targeting EGFRvIII and CD3 for the Treatment of Glioblastoma Multiforme. J Immunother Cancer. 2020 Nov;8(2):e001153.
Our products and services are for research use only, and not for use in diagnostic or therapeutic procedures.
Welcome! For price inquiries, we will get back to you as soon as possible.
To order, please email
INQUIRY


 Fig.1 Functional Domains of EGFR and EGFRvIII
Fig.1 Functional Domains of EGFR and EGFRvIII
 Fig.2 Signaling Pathways Mediated by EGFR/EGFRvIII (An Z, 2018)
Fig.2 Signaling Pathways Mediated by EGFR/EGFRvIII (An Z, 2018)
 Fig.3 Schematic Diagram of the Mechanism of BsAbs Targeting CD3 and EGFRvIII (Iurlaro R, 2022)
Fig.3 Schematic Diagram of the Mechanism of BsAbs Targeting CD3 and EGFRvIII (Iurlaro R, 2022)