What is Charge Pair Bispecific Antibody
Charge pair bispecific antibody (CPBA) is a novel type of bispecific antibody that can simultaneously recognize two different antigens or two different epitopes on the same antigen, thereby achieving various innovative biological effects, such as activating effector cells to kill tumor cells, replacing a missing link in an enzyme reaction, or acting as a molecular Trojan horse to enter hard-to-reach organelles, tissues and organs. Charge pair bispecific antibody uses charge pairs to achieve the correct pairing of two different antibody fragments, so it has high stability and purity, and is easy to express and purify. There are several methods for generating CPBA, including genetic engineering, chemical coupling and cell hybridization, but each method also has some limitations, such as efficiency, feasibility, cost and scalability. Charge pair bispecific antibody has a broad clinical application prospect, especially in the field of tumor immunotherapy. A few CPBA have been approved for marketing, and many CPBA are in different stages of clinical trials.
Structural Features of Charge Pair Bispecific Antibody
Charge pair bispecific antibody is composed of two different Fab fragments, each containing a heavy chain (HC) and a light chain (LC). The HC and LC of each Fab fragment are linked by a disulfide bond, and the two Fab fragments are connected by a hinge region. The key feature of CPBA is that the HC and LC of each Fab fragment are designed to have complementary charge pairs at their interface, which facilitate the correct pairing of the two HCs and prevent the formation of homodimers or unwanted heterodimers. The charge pairs are introduced by engineering specific amino acid residues at the CH3-CH3' interface of the Fc region of an antibody.
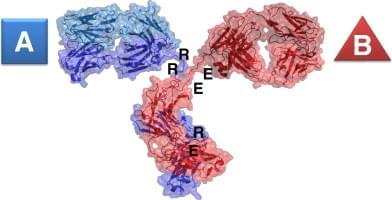
Fig.1 Diagram of a charge pair bispecific antibody (J Mol Biol, 2012).
The structural characteristics of CPBA affect its function, stability, expression and purification in several ways. First, the charge pairs enable CPBA to have comparable high affinity against antigens as its parental antibodies, as they do not interfere with the antigen-binding sites. Second, the charge pairs enhance the stability of CPBA by reducing aggregation and increasing thermal stability. Third, the charge pairs facilitate the expression and purification of CPBA by allowing efficient co-expression of four polypeptide chains in a single cell and selective purification by ion exchange chromatography. Fourth, the charge pairs allow CPBA to retain the Fc-mediated effector functions, such as antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC), which can be modulated by engineering other residues in the Fc region.
Generation Methods of Charge Pair Bispecific Antibody
There are several methods for generating CPBA, which can be classified into three main categories: genetic engineering, chemical coupling and cell hybridization. Each method has its own advantages and disadvantages, which will be discussed below.
Genetic engineering is the most widely used method for generating CPBA, as it allows precise control of the sequence and structure of the antibody molecules. There are two main approaches for genetic engineering of CPBA: co-expression and chain exchange. Co-expression involves co-transfection of two different antibody genes into a single host cell, which then co-expresses both antibodies and assembles them into CPBA. The correct pairing of the HCs is ensured by the charge pairs at the CH3-CH3' interface, while the correct pairing of the LCs is ensured by their natural specificity to their respective HCs. Co-expression can be achieved in various expression systems, such as mammalian cells, bacteria, yeast and plants. The advantages of co-expression are high efficiency, simplicity and scalability. The disadvantages are potential mispairing of HCs or LCs, low expression yield and high purification cost. Chain exchange involves expression of two different antibody genes in separate host cells, followed by mixing and reduction of the purified antibodies to induce chain exchange reactions. The chain exchange reactions are driven by the charge pairs at the CH3-CH3' interface, which favor the formation of heterodimers over homodimers or unwanted heterodimers. Chain exchange can also be achieved in various expression systems, but requires mild reduction conditions that do not affect the antigen-binding sites or the Fc-mediated effector functions. The advantages of chain exchange are high purity, high yield and low purification cost. The disadvantages are low efficiency, complexity and limited scalability.
Chemical coupling involves conjugation of two different antibody molecules or fragments by chemical cross-linkers, such as maleimide-thiol or aldehyde-hydrazide. The conjugation sites can be engineered at specific positions on the antibody molecules or fragments to avoid interference with antigen-binding sites or Fc-mediated effector functions. Chemical coupling can generate various formats of CPBA, such as Fab-Fab, scFv-scFv or IgG-IgG. The advantages of chemical coupling are flexibility, versatility and modularity. The disadvantages are potential loss of activity, stability or specificity due to chemical modification, heterogeneity of products and difficulty in purification.
Cell hybridization involves fusion of two different antibody-producing cells (such as hybridomas) to generate a quadroma cell that produces CPBA. The correct pairing of the HCs is ensured by the charge pairs at the CH3-CH3' interface, while the correct pairing of the LCs is ensured by their natural specificity to their respective HCs. Cell hybridization can generate IgG-like CPBA with intact Fc region and effector functions. The advantages of cell hybridization are simplicity and compatibility with existing hybridoma technology. The disadvantages are low efficiency, instability and heterogeneity of quadroma cells and products, and ethical issues related to animal use.
Clinical Applications of Charge Pair Bispecific Antibody
CPBA has a broad clinical application prospect, especially in the field of tumor immunotherapy, as it can simultaneously target two different antigens or epitopes on tumor cells or immune cells, and elicit various biological effects, such as tumor cell killing, immune cell activation, receptor blocking or signaling modulation. CPBA can also overcome some of the limitations of conventional monoclonal antibodies (MoAbs), such as antigen escape, low tumor penetration, low affinity or specificity, or resistance to Fc-mediated effector functions. CPBA can be divided into two categories according to their clinical status: approved CPBA and investigational CPBA.
So far, only three CPBA have been approved for marketing by the US Food and Drug Administration (FDA) or the European Medicines Agency (EMA). They are blinatumomab, catumaxomab and emicizumab. Table 1 summarizes their main characteristics and indications.
Table 1. Approved CPBA
|
Name
|
Target
|
Approval date
|
Indication
|
Population
|
Region
|
|
Blinatumomab
|
CD19/CD3
|
2014/2015
|
Acute lymphoblastic leukemia (ALL)
|
Relapsed/refractory B-cell precursor ALL
|
US/EU
|
|
Catumaxomab
|
EpCAM/CD3
|
2009
|
Malignant ascites
|
EpCAM-positive cancer with refractory ascites
|
EU
|
|
Emicizumab
|
Factor IXa/Factor X
|
2017/2018
|
Hemophilia A
|
Patients with factor VIII inhibitors
|
US/EU
|
Blinatumomab is a bispecific T-cell engager that binds to CD19 on B-cell malignancies and CD3 on T cells, and activates T cells to kill tumor cells. It is the first FDA-approved CPBA and the first bispecific T-cell engager for cancer treatment. It has shown remarkable efficacy and safety in patients with relapsed/refractory B-cell precursor ALL. Catumaxomab is a trifunctional antibody that binds to EpCAM on epithelial cancer cells, CD3 on T cells and FcγRI on accessory immune cells, and triggers a tri-cell complex formation that leads to tumor cell lysis and cytokine release. It is the first EMA-approved CPBA and the first trifunctional antibody for cancer treatment. It has shown significant improvement in puncture-free survival and quality of life in patients with EpCAM-positive cancer with refractory ascites. Emicizumab is a bispecific antibody that mimics the function of factor VIII by bridging factor IXa and factor X, and restores the coagulation cascade in patients with hemophilia A . It is the first FDA-approved CPBA for non-cancer indication and the first bispecific antibody for hemophilia treatment. It has shown remarkable reduction in bleeding events and improvement in quality of life in patients with hemophilia A with factor VIII inhibitors.
There are more than 110 CPBA in various stages of clinical trials for different indications, mainly for cancer treatment. They can be classified into different types according to their formats, targets or mechanisms of action. Table 2 summarizes some of the representative examples of investigational CPBA.
Table 2. Investigational CPBA
|
Name
|
Type
|
Target
|
Mechanism of action
|
Clinical stage
|
|
ZW25/ZW49
|
IgG-like CPBA
|
HER2/HER2
|
Dual-epitope binding and signaling blockade
|
Phase 2/Phase 1
|
|
REGN1979/ REGN5458/ REGN4018
|
IgG-like CPBA
|
CD20/CD3/ BCMA/CD3/ MUC16/CD3
|
T-cell redirection and tumor cell killing
|
Phase 2/Phase 1/Phase 1
|
|
Mosunetuzumab/ Epcoritamab/ Glofitamab/ Cusatuzumab
|
Fc-containing bispecific T-cell engagers
|
CD20/CD3/ CD3/CD20/ CD70/CD3/ CD20/CD3/ CD38/CD3
|
T-cell redirection and tumor cell killing
|
Phase 2/Phase 1/Phase 1
|
|
Duvelisib/ Cosibelimab/ Talquetamab/ Teclistamab/ AMG 701
|
IgG-like CPBA with Fc mutations or modifications
|
PI3Kδ/PI3Kγ/ PD-L1/GPRC5DxCD3/ BCMAxCD3/BCMAxTCR activator (TA) domain
|
Signaling modulation or T-cell activation and tumor cell killing
|
Phase 2/Phase 1
|
|
AMG 420/ AMG 596/ AMG 199/ AMG 160/ AMG 757/ AMG 119
|
Bispecific T-cell engagers without Fc region
|
BCMAxCD3 /CD33xCD3 /EGFRvIIIxCD3 /MUC17xCD3 /PSCAxCD3 /DLL3xCD3 /ROR1xCD3
|
T-cell redirection and tumor cell killing
|
Phase 1
|
|
Rovalpituzumab tesirine/ Loncastuximab tesirine/ Belantamab mafodotin/ Cusatuzumab/ Duvelisib/ Cosibelimab/ Talquetamab
|
Antibody-drug conjugates (ADCs) or antibody-radionuclide conjugates (ARCs)
|
DLL3/pyrrolobenzodiazepine (PBD) dimer /CD19/pyrrolobenzodiazepine (PBD) dimer /BCMA/maytansinoid /CD70/calicheamicin /PI3Kδ /PI3Kγ /PD-L1/GPRC5D /actinium-225 (Ac-225) or lutetium-177 (Lu-177)
|
Targeted delivery of cytotoxic agents to tumor cells or tissues
|
Phase 2
|
Conclusion
CPBA is a novel type of bispecific antibody that can simultaneously recognize two different antigens or epitopes, and achieve various innovative biological effects. CPBA has several advantages over conventional monoclonal antibodies, such as higher affinity, specificity, stability and purity, as well as retained Fc-mediated effector functions. CPBA can be generated by different methods, such as genetic engineering, chemical coupling and cell hybridization, each with its own strengths and weaknesses. CPBA has a broad clinical application prospect, especially in the field of tumor immunotherapy, where it can target multiple tumor-associated antigens or immune checkpoints, and elicit potent anti-tumor responses. CPBA is a promising therapeutic modality that may offer new solutions for the treatment of complex diseases.
References
1. Estes B, et al. Next generation Fc scaffold for multispecific antibodies. iScience. 2021 Nov 15;24(12):103447.
2. Ma J, et al. Bispecific Antibodies: From Research to Clinical Application. Front Immunol. 2021 May 5;12:626616.
3. Wang Q, et al. Design and Production of Bispecific Antibodies. Antibodies (Basel). 2019 Aug 2;8(3):43.
4. Dengl S, et al. Format chain exchange (FORCE) for high-throughput generation of bispecific antibodies in combinatorial binder-format matrices. Nat Commun. 2020 Oct 2;11(1):4974.
5. Wang ZL, et al. Bispecific antibodies for cancer immunotherapy: Current perspectives. Biomark Res. 2019 Oct 22;7(1):25.
6. Kontermann RE. Dual targeting strategies with bispecific antibodies. mAbs . 2012 Mar-Apr;4(2):182–197.
7. Spiess C, et al. Alternative molecular formats and therapeutic applications for bispecific antibodies . Mol Immunol . 2015 Apr;67(2 Pt A):95–106 .
8. Brinkmann U, et al. The making of bispecific antibodies . mAbs . 2017 Feb-Mar;9(2):182–212 .
9. Fan G, et al. Bispecific antibodies and their applications . J Hematol Oncol . 2015 Dec 29;8(1):130 .
10. Pavel Strop, et al. Generating bispecific human IgG1 and IgG2 antibodies from any antibody pair. J Mol Biol. 2012 Jul 13;420(3):204-19.
Our products and services are for research use only, and not for use in diagnostic or therapeutic procedures.
Welcome! For price inquiries, we will get back to you as soon as possible.
To order, please email
INQUIRY