What is κλ-body
κλ-bodies are fully human, non-engineered bispecific antibodies that consist of one heavy chain and two different light chains, one κ and one λ. They have high affinity, stability, expression and purification properties. The concept of κλ-bodies was first proposed by Fischer et al.in 2015, who demonstrated that light chains can play a dominant role in mediating specificity and high affinity for two different targets. They also showed that κλ-bodies can support multiple modes of action, such as dual blockade, cross-linking or recruitment of effector cells. Moreover, they revealed that κλ-bodies have the same stability and pharmacokinetic properties as therapeutic antibodies, and can be produced at the industrial scale using a platform process. Since then, several κλ-bodies have been developed and tested for various indications, especially in oncology.
κλ-bodies represent a unique, fully human format that exploits light-chain variable domains for antigen binding and light-chain constant domains for robust downstream processing. They retain the native structure of human IgG, without any modification or engineering. This confers them several advantages over other bispecific antibody formats, such as full human origin, natural assembly, diverse mechanisms of action, low immunogenicity, etc. However, they may also face some challenges or limitations, such as light chain exchange, target selection, dose optimization, etc. In summary, κλ-bodies are a novel class of bispecific antibodies that leverage the power of light chains to achieve dual specificity and high affinity. They offer a simple and scalable way to produce native human bispecific IgG at the industrial scale. They have shown promising results in preclinical and clinical studies, especially in the field of immunotherapy.
Structural Features of κλ-body
A κλ-body is composed of two identical heavy chains and two different light chains, one κ and one λ.The heavy chain is common to both antigen-binding sites, while the light chains are responsible for providing the distinct specificities.The κ and λ light chains are co-expressed and assembled with the heavy chain in a single cell, resulting in a mixture of25% monospecific κκ-bodies, 25% monospecific λλ-bodies and 50% bispecific κλ-bodies.The bispecific κλ-bodies can be easily purified from the supernatant using affinity reagents that bind to the two different light chains.
A κλ-body retains the native structure of human IgG, without any modification or engineering. It has the same Fc region as a conventional antibody, which can mediate effector functions such as antibody-dependent cellular cytotoxicity (ADCC) or complement-dependent cytotoxicity (CDC). It also has the same hinge region as a conventional antibody, which allows flexibility and optimal orientation of the antigen-binding sites. Moreover, it has two different light-chain constant domains (CL), which confer stability and solubility to the antibody, and facilitate its downstream processing.
Discovery and Optimization Methods
Phage display technology is a widely used method for antibody discovery and engineering. It involves displaying antibody fragments (such as Fab or scFv) on the surface of bacteriophages, and selecting them by binding to a target antigen. To discover κλ-bodies, phage display technology can be used to generate κ and λ light chain libraries, each containing a large diversity of light-chain variable domains. The libraries can be co-expressed with a common heavy chain on phages, and panned against two different targets to isolate bispecific κλ-bodies.
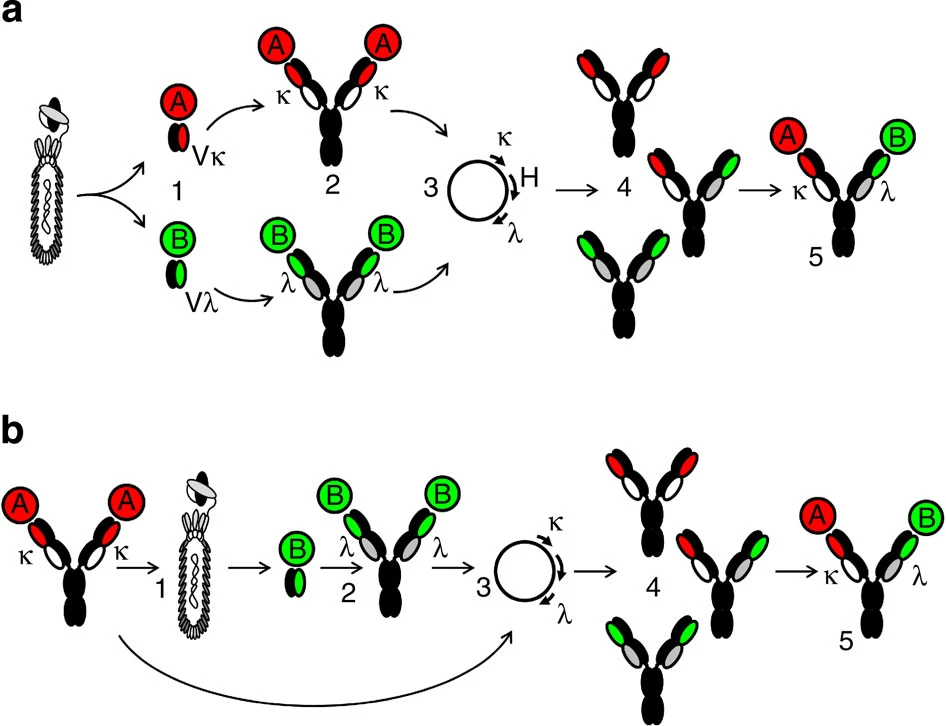
Fig.1 Approaches for the generation of bispecific IgG based on light-chain diversity. (Fischer N, 2015)
Transgenic mouse technology is another method for antibody discovery and engineering. It involves generating mice that carry human antibody genes, and immunizing them with a target antigen to elicit an immune response. To discover κλ-bodies, transgenic mouse technology can be used to generate mice that express human κ and λ light chains, along with a common human heavy chain. The mice can be immunized with two different targets, and their spleen cells can be harvested to isolate bispecific κλ-bodies.
Once the κλ-bodies are discovered, they can be further optimized by various methods, such as affinity maturation, humanization, sequence optimization, etc. These methods aim to improve the binding properties, stability, expression and solubility of the κλ-bodies, as well as to reduce their immunogenicity and aggregation potential.
Advantages and Disadvantages of κλ-body
κλ-bodies have several advantages over other bispecific antibody formats. First, they are fully human, which reduces the risk of immunogenicity and increases the compatibility with human Fc receptors. Second, they retain the native structure of human IgG, without any modification or engineering. This confers them high stability, solubility and pharmacokinetic properties. Third, they support multiple modes of action, such as dual blockade, cross-linking or recruitment of effector cells. This enables them to target different types of antigens and modulate different immune pathways. Forth, κλ-body can be produced by co-expressing one heavy chain and two different light chains in stably transfected Chinese hamster ovary (CHO) cells, followed by a downstream purification process that is applicable to any κλ-body. This avoids the need for extensive engineering or foreign sequences that are often required for other bispecific antibody formats.
However, κλ-bodies may also have some drawbacks. For example, κλ-body may be susceptible to light chain exchange with endogenous antibodies in vivo, resulting in loss of bispecificity and potential formation of unwanted antibodies. This can be mitigated by introducing mutations in the CH1 domain of the heavy chain or the CL domain of the light chains to prevent light chain swapping. In addition, κλ-body requires careful selection of targets that are expressed on the desired cells and not on normal cells, to avoid off-target effects or toxicity. For example, CD47 is widely expressed on both cancerous and non-cancerous cells, and blocking it may cause depletion of red blood cells. This can be overcome by using strategies such as masking, affinity tuning, or conditional activation of the antibody. What’s more, κλ-body may have different optimal doses depending on the targets, the mode of action, and the pharmacokinetics of the antibody. For example, TG-1801 may require higher doses than conventional antibodies to achieve sufficient occupancy of both CD47 and CD19 on tumor cells. This can be addressed by conducting dose-finding studies and pharmacodynamic analyses in preclinical and clinical settings.
Clinical Data of κλ-body
There is only one κλ-body that has been approved for marketing so far. It is TG-1801 (formerly NI-1701), a κλ-body that targets CD19 and CD47, two important antigens involved in B cell malignancies. CD19 is a B cell surface marker that is widely expressed in various B cell lymphomas and leukemias. CD47 is a “don’t eat me” signal that protects tumor cells from phagocytosis by macrophages. TG-1801 can simultaneously bind to CD19 and CD47, and induce dual blockade of these two targets, as well as cross-linking of tumor cells and macrophages. This results in enhanced tumor cell killing by phagocytosis and antibody-dependent cellular cytotoxicity (ADCC). TG-1801 was approved by the US Food and Drug Administration (FDA) in December 2020 for the treatment of relapsed or refractory diffuse large B cell lymphoma (DLBCL), under the accelerated approval program. It is the first and only bispecific antibody approved for this indication. The approval was based on the results of a phase I dose-escalation trial (NCT03804996), which enrolled 44 patients with relapsed or refractory B cell lymphoma. The trial showed that TG-1801 had an overall response rate (ORR) of 43.6%, with a complete response rate (CRR) of 17.9%. The median duration of response (DOR) was 10.25 months. The most common adverse events (AEs) were cytokine release syndrome (CRS), neutropenia, anemia and infusion-related reactions (IRR). The AEs were mostly mild to moderate in severity, and manageable with standard interventions. TG-1801 is currently being evaluated in a phase II trial (NCT04108195), which aims to enroll 100 patients with relapsed or refractory DLBCL. The trial has two cohorts: one for patients who are eligible for autologous stem cell transplant (ASCT), and another for patients who are ineligible for ASCT. The primary endpoint is ORR, and the secondary endpoints include CRR, DOR, progression-free survival (PFS), overall survival (OS) and safety. The trial is expected to be completed by December 2023.
Besides TG-1801, there are several other κλ-bodies in preclinical or clinical development for various indications, especially in oncology. For example, NI-1702 is a κλ-body that targets CD38 and CD47, two antigens involved in multiple myeloma (MM) and other hematological malignancies. CD38 is a transmembrane glycoprotein that is highly expressed on MM cells and plays a role in cell proliferation, survival and drug resistance. NI-1702 can simultaneously bind to CD38 and CD47, and induce dual blockade of these two targets, as well as cross-linking of tumor cells and macrophages.
Table 1. Overview of κλ-bodies products in development
|
κλ-body
|
Targets
|
Trial Number
|
Trial Phase
|
Trial Design
|
Primary Endpoints
|
Clinical Progress and Expected Outcomes
|
|
NI-1702
|
CD38 and CD47
|
NCT04649359
|
I/IIa
|
Open-label, dose-escalation and expansion study
|
Safety, tolerability, pharmacokinetics, pharmacodynamics and preliminary efficacy of NI-1702 in patients with relapsed or refractory multiple myeloma (MM) or other CD38-positive hematological malignancies
|
The trial started in December 2020 and is expected to enroll 90 patients; the trial is currently recruiting participants2
|
|
NI-1703
|
CD40 and 4-1BB
|
NCT04722146
|
I/IIa
|
Open-label, dose-escalation and expansion study
|
Safety, tolerability, pharmacokinetics, pharmacodynamics and preliminary efficacy of NI-1703 in patients with advanced solid tumors or B cell lymphomas that express CD40
|
The trial started in January 2021 and is expected to enroll 90 patients; the trial is currently recruiting participants2
|
|
NI-1801 (formerly TG-1802)
|
CD20 and CD47
|
NCT03804996 (TG-1801) and NCT04722133 (NI-1801)
|
I/IIa (TG-1801) and I/IIa (NI-1801)
|
Open-label, dose-escalation and expansion study (TG-1801); open-label, dose-escalation and expansion study with a randomized phase II component (NI-1801)
|
Safety, tolerability, pharmacokinetics, pharmacodynamics and preliminary efficacy of TG-1801 or NI-1801 in patients with relapsed or refractory B cell lymphoma or chronic lymphocytic leukemia (CLL) that express CD20; comparison of the efficacy of NI-1801 versus TG-1801 in patients with relapsed or refractory DLBCL that express CD20 (NI-1801 only)
|
The trial of TG-1801 started in January 2019 and is expected to enroll 100 patients; the trial is currently active but not recruiting participants1; the trial of NI-1801 started in January 2021 and is expected to enroll 240 patients; the trial is currently recruiting participants2
|
In summary, κλ-bodies are a new dimension in terms of therapeutic potential, while exploiting the beneficial structural characteristics of monoclonal antibodies. They provide physicians with powerful approaches to effectively treat a diverse range of medical conditions, especially in immunotherapy. They also require further investigation and optimization to overcome some hurdles and improve their performance and safety.
References
1. Fischer N, et al. Exploiting light chains for the scalable generation and platform purification of native human bispecific IgG. Nat Commun. 2015 Feb 12;6:6113.
2. Light Chain Bioscience. Next-generation immunotherapy with native bispecific antibodies. Nat Biopharma Dealmakers. 2020;2020:16-17.
3. Magistrelli G, et al. Optimizing assembly and production of native bispecific antibodies by codon de-optimization. MAbs. 2017 Feb/Mar;9(2):231-239.
4. Elshiaty M, et al. Principles and Current Clinical Landscape of Multispecific Antibodies against Cancer. Int J Mol Sci. 2021 May 26;22(11):5632.
5. Acheampong DO. Bispecific Antibody (bsAb) Construct Formats and their Application in Cancer Therapy. Protein Pept Lett. 2019;26(7):479-493.
6. Dheilly E, et al. Selective Blockade of the Ubiquitous Checkpoint Receptor CD47 Is Enabled by Dual-Targeting Bispecific Antibodies. Mol Ther. 2017 Feb 1;25(2):523-533.
7. Magistrelli G, et al. Tuning Relative Polypeptide Expression to Optimize Assembly, Yield and Downstream Processing of Bispecific Antibodies. Antibodies (Basel). 2018 Aug 10;7(3):29.
8. Kaur S, et al. Mucins in pancreatic cancer and its microenvironment. Nat Rev Gastroenterol Hepatol. 2013;10(10):607-20.
9. Brinkmann U, et al. The making of bispecific antibodies. MAbs. 2017;9(2):182-212.
10. Kontermann RE. Bispecific antibodies for cancer immunotherapy: current perspectives. BioDrugs. 2019;33(4):347-361.
Our products and services are for research use only, and not for use in diagnostic or therapeutic procedures.
Welcome! For price inquiries, we will get back to you as soon as possible.
To order, please email
INQUIRY